Abstract 
Restoration projects should follow four steps: identify and understand the threat; remove or reduce the threat; monitor the effect; intervene further when necessary. "Restoration" is sometimes thought of as the last of these four, but restoration may often be achieved by the first three alone. The last step implies a repeat cycle of the first three, identifying and dealing with additional threats. A first cycle usually has a community focus (for monitoring and ecological studies), whereas subsequent cycles usually imply a species focus. The Dry Zone is the most widely distributed of the Galapagos vegetation zones. It has been damaged on several islands, mainly by introduced herbivores and plants. Restoration following damage by introduced species (on which this paper focuses) differs from that after habitat destruction, in that the less intervention may be required, but the precise intervention required may be less clear. Three options for removal of an introduced species threat are manual-chemical control, biological control, and eradication. The cost distributions of these options are explored with examples. Galapagos data show that removal of an introduced invasive plant or animal often results in rapid regeneration of native vegetation, as revealed by monitoring, even after decades of damage. However, sometimes some species do not regenerate adequately after removal of the threat which caused their decline. In such cases further action (step 4) is required to achieve their restoration. Four Galapagos examples illustrate the kind of intervention, including further research, required for different species.
Key words: Galapagos, Vegetation, Restoration, Invasive Species
Resumen 
Los proyectos de restauración deben seguir cuatro pasos: identificar y entender la amenaza; remover o reducir la amenaza; monitorear el efecto; intervenir más cuando sea necesario. "Restauración" a veces se considera el último de estos pasos, pero en muchos casos se puede lograr restauración con solo la implementación de los primeros tres. El último paso implica un nuevo ciclo de los primeros tres, identificando y tratando nuevas amenazas adicionales. El primer ciclo suele tener un enfoque en la comunidad vegetativa (para los estudios de monitoreo y ecología), mientras ciclos adicionales suelen implicar un enfoque a nivel de especie. La Zona Seca es la zona de vegetación más amplia de Galápagos. Se encuentra dañada en varias de las islas, principalmente debido a los mamíferos introducidos y plantas introducidas. Restauración luego de daño causado por especies introducidas (el enfoque de este artículo) es diferente de luego de destrucción de hábitat, en que puede requerir menos intervención, pero la intervención precisa requerida puede ser menos evidente. Tres opciones para remover una especie introducida son control manual-químico, control biológico, y erradicación. Se presentan ejemplos de la distribución de los costos de estas tres. Datos de Galapagos demuestran que el remover una planta o animal introducido suele resultar en la regeneración rápida de la vegetación nativa, revelada por el seguimiento, aun después de décadas de daño. Sin embargo, a veces ciertas especies no se recuperan adecuadamente después de la eliminación de la amenaza que causó su disminución. En estos casos se requiere acción específica (paso 4) dirigida a la restauración de ellas. Cuatro ejemplos demuestran la clase de intervención, incluyendo más investigación, necesaria para diferentes especies.
Introduction 
What
is restoration when applied to dry-zone vegetation in Galapagos?
In this paper I will argue that restoration should be seen as one element in a strategy for conservation management, and use examples from Galapagos to illustrate such a strategic approach to restoration of dry forest and other semi-arid vegetation types in the islands. The main questions I address are:
What can we learn about vegetation restoration from Galapagos experiences?
What should we do to ensure recovery from a situation of threat/degradation?
I argue that four basic steps should be followed in a specific sequence, in order to ensure restoration of damaged dry forest and other habitats. These are:
1. Identify and understand the threat and its effects: what has caused the habitat change and how has it changed?
2. Remove or reduce the threat.
3. Monitor the effects of removing or reducing the threat.
4. Intervene further only when necessary to ensure recovery.
It is the last of these four steps which is often thought of as "restoration", but I argue that the whole process of steps 1-4 should be seen as restoration and that step 4 may frequently not be required. Further, when it is required, Step 4 usually necessitates more research (return to Step 1), to identify and understand additional threat factors which need to be counteracted, and to determine life cycle stages at which the additional interventions (Steps 2-3) may need to be made. That is to say, Step 4 is simply a renewed cycle of Steps 1-3.
DRY
ZONE VEGETATION IN GALAPAGOS
The vegetation of Galapagos is determined largely by orogenic rainfall and is therefore strongly zoned by altitude (Wiggins & Porter 1971). Although other schemes have been used, I accept here five principal zones, each of which can be divided into sub-zones based mainly on habitat structure (e.g. forest, shrubland or herb-dominated communities). The lowest of the five principal zones is the Littoral Zone, a narrow fringe determined by proximity to the sea and the influence of salt spray. Above this, the Dry Zone of scrub and dry woodland extends uphill, where it blends into a Transition Zone of closed forest. Above this is the Humid Zone, and finally on the highest islands, above the main cloud layer, a second High-altitude Dry Zone.
The Galapagos archipelago comprises 14 principal islands and more than 120 smaller islets, where an island or islet may be defined as any landmass permanently isolated (at all tide stages) by sea and capable of supporting terrestrial vegetation (which excludes mangroves). All Galapagos islands have a Littoral Zone, and some 50 islets are so small and low that they are entirely Littoral Zone. All remaining islands, i.e. about 80, have a Dry Zone, whereas only seven islands are high enough to carry a Humid Zone and only two are high enough to carry a High-altitude Dry Zone. The Dry Zone thus accounts for the largest total land area of any of the main Galapagos vegetation zones (Fig. 1).
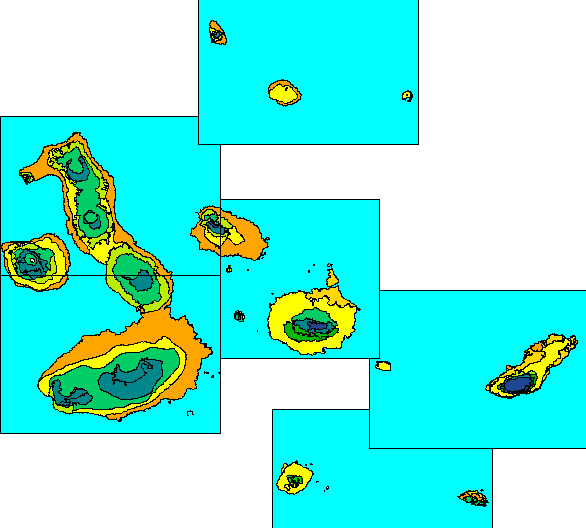
Figure 1. Dry Zone (yellows and oranges), Transition
Zone (greens) and Humid Zone (blues) of the central Galapagos
archipelago; High-altitude Dry Zone not distinguished on this map.
Ecological zone map of PRONAREG et al. (1987).
The vegetation formations of the Dry Zone include various open woodland communities, the most common being dominated by Bursera graveolens. Other communities include more or less wooded shrubland dominated by different species in different sites, including Cordia lutea, Gossypium darwinii, Opuntia spp. or Croton scouleri, among others. Other areas carry only sparse low shrubs and annual herbs, while others are open lava with scattered annuals or cacti. The Transition Zone is also semideciduous mixed-species Dry Forest (Bosque Seco Premontano).
The Problem
Threats
to Galapagos vegetation
Galapagos vegetation has suffered three main threats, direct habitat destruction by man, direct exploitation of certain species, and introduced species.
Historically, direct habitat destruction by man has been important, with the creation of large agricultural areas in the highlands of four islands, and settlements on the coasts of five. The lowland towns have damaged only a tiny proportion of the widespread Dry Zone. In contrast, the agricultural areas have destroyed large proportions of the Humid Zone of Floreana, Santa Cruz and San Cristóbal, and of Sierra Negra volcano on Isabela, and have also damaged substantial proportions of the Transition Zone on these islands (e.g. Fig. 2; Snell et al. 2002/in press). There is currently pressure and legal provision to take more land from the Galapagos National Park for development in Galapagos. Depending where this is done, it could have major or lesser effects on one or more vegetation zones in the coming years.
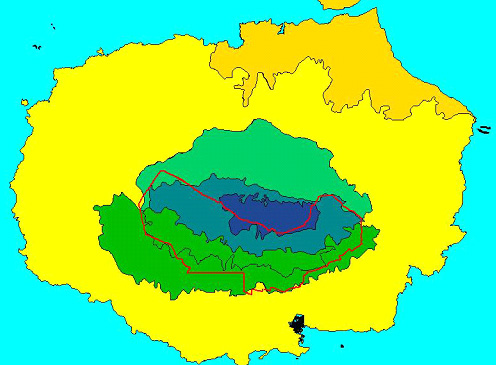
Figure 2. Impact of the conversion of natural habitats
to agricultural (red outline) and urban (town on south coast) use on
Santa Cruz Island. Habitat zones as in Fig. 1.
Direct exploitation, although an important cause of declines and even extinctions of some Galapagos animal species, such as giant tortoises and sea cucumbers, has affected only a few plants. Some native and endemic trees have been exploited for their timber, which may have caused population structural changes, and in one case, the Floreana endemic tree Lippia salicifolia, probably contributed to its current threatened status (Mauchamp et al. 1998; Tye 2002). However, direct exploitation has not contributed significantly to changes in the structure or composition of the Dry or Transition Zones, except very locally where mature Piscidia carthagenensis trees have been over-exploited.
Introduced species are currently regarded as the main threat to the biological diversity of Galapagos (Bensted-Smith 2002/in press). Invasive introduced species may be regarded as having two main effects: competition with native species and predation on them. In the case of effects on vegetation, predation is equated with herbivory, while the main competitors for native plants are invasive introduced plants (Fig. 3ab).

Figure 3a. Effects of competition (invasion of the tree Cinchona pubescens) in the naturally treeless highlands of Santa Cruz Island: photo H. Jäger).

Figure 3b. Effects of predation (= herbivory, destruction of dry forest on Alcedo Volcano by goats) by introduced species in Galapagos.
Restoration
required following different kinds of damage
From the above it will be seen that each of the three main threats leads to a distinct restoration requirement. Habitat destruction, conversion to agricultural or urban use, may require considerable investment in order to restore native vegetation, after any decision has been made to remove the threat (i.e. to cease use and restore native habitat). Most native plant species will have been eliminated from such areas, so their return following cessation of agriculture, or dis-occupation of the area, is highly unlikely, at least in a reasonable time frame. Restoration following such use therefore implies intervention to restore the entire plant community. Such restoration has not been attempted on a large scale in Galapagos, although pilot projects have been started in recent years in the agricultural zones, where some land-owners have begun conservation-restoration projects in part because of their potential value as an ecotourism attraction. However, these projects all focus on humid-zone vegetation, although some include upper Transition Zone areas, and none affects the Dry Zone.
In contrast, restoration following direct exploitation of individual species would require intervention focused on those threatened species. So far, there have been no attempts in Galapagos to restore the populations of any timber tree whose populations have been affected by over-exploitation. Management interventions have simply comprised attempts to reduce use.
Restoration following damage by introduced species is more complex, in that the level of intervention required (community vs. individual species) is intuitively less clear. However, restoration following damage by introduced species is precisely the area where most research has been done in Galapagos. Experiences and lessons learnt in Galapagos may thus provide useful insights into the factors that need to be taken into account when planning a restoration programme following this kind of damage. This kind of restoration programme is the focus of the rest of this paper.
Restoration steps following damage caused by competition from Invasive Plants
Step
1: Identify and understand the threat.
Although questions such as "what damage are invasive plants causing?", are also important in order to permit evaluation of the success of a restoration programme (reversal of the changes), the principal question to which an answer is required for planning restoration following damage caused by invasive plants is "which species are causing most damage?". From the point of view of competition, invasive plant species may be classed as either "integrators" or "transformers" (see Richardson et al. 2000). Integrators, often but not always small herbaceous species, may invade natural communities without dominating them or displacing the majority of the native vegetation. They are thus often difficult to control, but they also cause lesser effects. Transformers in contrast are often large woody species, or herbaceous species that can form dense stands, such as grasses. They may thus displace the majority of the native vegetation and can replace it with a totally alien community, in terms of both structure (e.g replacing herbaceous communities with forest) and species composition (displacing native species).
In the Humid Zone, many invasive plant species have escaped from the agricultural areas and are causing widespread, landscape-scale changes to the vegetation. In the Dry Zone, invasive plants have not yet caused serious damage, but they may do so in the future, as many potentially invasive introduced species that are adapted to drier conditions have been introduced as garden ornamentals and are grown in the lowland towns. However, some invasive plants, such as Cedrela odorata, Psidium guajava, Passiflora edulis and a variety of grasses, are invading the Transition Zone on the inhabited islands, spreading downslope from the agricultural areas and replacing native dry forest. Monitoring projects and ecological studies help to determine which invasive plants are transformers. For example in the mixed forest of the Transition Zone of Santa Cruz island, a 30-year monitoring programme of permanent plots has revealed the complete replacement of the dominant tree Scalesia pedunculata by the introduced Cedrela odorata and Psidium guajava within a 13-year period (1992-2004: Fig. 4ab).

Figure 4a. Replacement of Transition Zone Scalesia
pedunculata forest by introduced Cedrela odorata and Psidium guajava. 1992. Photos: O. Hamann.

Figure 4b. Replacement of Transition Zone Scalesia
pedunculata forest by introduced Cedrela odorata and Psidium guajava. 2004. Photos: O. Hamann.
Step
2: Remove or reduce the threat.
The science of invasive plant management is still relatively young, and there are few examples of control of a plant invasion on a large scale in Galapagos so far. In the case of the transformation of the Transition Zone forests, a pilot project was undertaken to kill all Cedrela trees over several hectares of heavily invaded forest. Two major treatments were used, one where trees were felled and stumps killed by painting herbicide on them, and the other where trees were killed standing by injecting herbicide into machete cuts in the trunks. The latter treatment avoided damaging the understorey caused by felling trees, as the understorey included young plants of many native canopy species. Both trials gave almost 100% control of Cedrela, resulting in opening of the canopy, which had become dominated bythis species. This was followed by strong regrowth of native vegetation, but also by regeneration of Cedrela from seed (Fig. 5ab), indicating that follow-up treatment would be required to ensure full restoration of native forest. However, the trials indicated that it would be relatively easy to achieve restoration over large areas, if sufficient funding could be found to employ a small team to carry out initial control and follow-up maintenance.

Figure 5a. Former Transition Zone forest dominated by
the invasive Cedrela odorata (the tall trees).

Figure 5b. Former Transition Zone forest dominated by
the invasive Cedrela odorata, following Cedrela control. The red oval indicates Cedrela regrowth, among a general regeneration of native species.
Larger projects are therefore needed in Galapagos to attack some of the worst invaders, and the key to success of a large project is detailed planning and a systematic approach. The best example of such an approach for planning a large-scale control, or even total eradication, project in Galapagos concerns Red Quinine, Cinchona pubescens, which has invaded some 12,000 ha on Santa Cruz island, mostly in the humid highlands but also extending to the Transition Zone.
If we are to begin such a project with a high probability of succeeding, we need to understand in great detail the biology of the target plant. This project illustrates the deeper level of understanding required at Step 1, once a target has been chosen. In the case of Cinchona, studies have been carried out in Galapagos on its reproductive cycle, seed dispersal and seedbank longevity (Rentería 2002) as well as on control trials, the impacts of the invasion and the impacts of control techniques (Jäger 1999). The biological studies are necessary to enable a control project to interrupt the reproductive cycle, prevent further seed production and permit steady reduction in the population of juvenile plants (before they reach reproductive age). Further studies have been carried out to estimate the cost of control at different densities (Buddenhagen & Yánez 2005). Since the main determining factor for the success of such a project is the availability of funds, it is important to be able to predict the total cost of a project, especially if the goal is total eradication from an island, in advance of beginning the attempt. All of these studies contribute to a management plan for either total eradication of the species from Santa Cruz or its control (reduction to low density) over the whole of the invaded area.
Management options for removing or reducing the threat caused by an invasive plant fall into three main categories:
1. "Classical" control, implying manual or chemical control where the aim is a reduction in density of the target species over a defined area, in practice usually specific sites considered to be of high biodiversity value.
2. Eradication, where the aim is complete removal of a species from a defined area, and where the chance of re-invasion is small. In practice this usually means eradication from an island.
3. Biological control, where the aim is permanent reduction in density over the entire invaded area.
Each of these options carries advantages and disadvantages, and cost distributions differ significantly between the three. In the case of classical control, costs are primarily determined by the size of the selected control area, which may in practice be determined by the size of the annual budget assigned by the management authority. This results in apparently lower costs, when compared with eradication or biological control, but the disadvantage is that success is variable from year to year (depending on variations in annual budget) and the investment never ends, since the species requires continued control year after year in order to maintain gains obtained by reducing the population of the target species. In the case of Cinchona on Santa Cruz, a suitable level of investment required to keep the most important sites on the island relatively free of the invader would be between US$100,000 and $300,000 per year (depending on the size of the area selected for treatment), permanently.
Biological control has a very different cost distribution, with initial investment high, being mainly the costs of the research required to identify an effective natural enemy, usually in the area of origin of the invader, and further research needed to determine that the identified agent will not have adverse effects on native plants or animals (specificity testing). However, once implemented, if control is successful, future costs reduce to near zero (low-frequency monitoring to ensure that control remains effective). The disadvantage associated with a new biocontrol project is that, until the research has been done and the release of the agent has been carried out, the ability to predict success is poor: an agent may or may not be found, and the agent may or may not reduce the target species' population to acceptable levels. In the case of Cinchona, such a project would be much cheaper than most biocontrol efforts, since it could be carried out entirely within-country, as the natural range of the species includes mainland Ecuador. The total cost would be c. $300,000.
Eradication, in contrast, may appear extremely expensive, since initial costs, as in biocontrol, are high, in this case not for the research, but for the initial knock-down of the population to interrupt the breeding cycle. However, as in the case of biocontrol, once the plant has been eradicated, costs reduce to near-zero, and a further advantage of eradication is that our ability to predict success is high, as long as the preliminary studies have been thorough. Predicting success largely comes down to predicting cost, the question then reducing to ability to find and dedicate the funds required, and to guarantee continuation of the project for the period required to achieve eradication. In the case of Cinchona, the cost of attempting an eradication from Santa Cruz with a high probability of success would be c. $6 million (cf. Buddenhagen & Yánez 2005), spread over 10 years, with about half the total budget being spent in the first three years (initial removal of all seed-producing trees over the entire invaded area).
Although eradication may thus appear prohibitively expensive, it may be the preferred option, given that future costs are effectively zero. It is far easier to guarantee a one-off investment of $6 million than to guarantee a defined level of funding for classical control, every year, for ever. Similarly, on economic grounds, biological control is always a cost-effective option for dealing with a serious, widespread invasion.
Restoration steps following damage caused by Herbivory
Step
1: Identify and understand the threat.
It is sometimes very easy to detect the effects of introduced herbivores on native plant communities and individual species. Introduced mammalian herbivores, especially feral goats Capra hircus, are notorious for their dramatic effects on oceanic islands (Baker & Reeser 1972; Breckon 2000; Coblentz 1978; Courchamp et al. 2003; Gould & Swingland 1980; Lever 1994), and are often blamed for vegetation change based on anecdotal (but obvious) evidence. Effects such as extinctions, rarity and changed species composition have been inferred based on qualitative comparisons with old accounts or photographs, while monitoring studies have examined regeneration following goat exclusion or eradication (e.g. Baker & Reeser 1972; Loope & Scowcroft 1985; North & Bullock 1986; all studies cited by Coblentz 1978). In Galapagos, Hamann (1975, 1979, 1993, 2001) documented goat damage on Santa Fe and Pinta islands, and De Vries & Calvopiña (1977) on Santiago. All these studies demonstrate dramatic changes in communities, and declines in individual species caused by goats.
Step
2: Remove or reduce the threat.
Solutions to such problems include protection, such as constructing exclosures around threatened communities or remnant populations of individual species. Both community- and species-focused fencing has been used in Galapagos. Fences were built around remnant vegetation communities on Santiago Island during the period 1973-1998, when goats devastated the vegetation throughout the island. Fences have also been constructed to protect populations of threatened plant species, such as Linum cratericola on Floreana Island, Cyathea weatherbyana on Alcedo Volcano of Isablea Island, and Scalesia retroflexa on Santa Cruz.
The advantage of fencing is that it is relatively easy, quick and cheap, but can only be used (at least while remaining cheap) to protect relatively small areas. Over larger areas other alternatives become more cost-effective, including control or eradication of the herbivore. As in the case of invasive plants, there are three management options for introduced herbivores: "classical" control (e.g. hunting), biological control and eradication.
As with plants, classical control can have lower annual costs, but is for ever, and success is variable, depending on variations in annual budget and priorities. Biological control has a high initial cost but later falling to near-zero, success prediction is uncertain, and biocontrol is in practice useful for only invertebrate animals. Eradication costs are initially high, but then reduce to near-zero (monitoring for reintroduction), and the prediction of success is high, at least for mammalian herbivores.
As an example, goats have been introduced to 12 Galapagos islands, and have so far been eradicated from seven of these (Fig. 6). Eradication projects are almost complete for one more (Santiago) and for the northern part of Isabela. Our successes are improving, with ever larger islands being freed from goats.
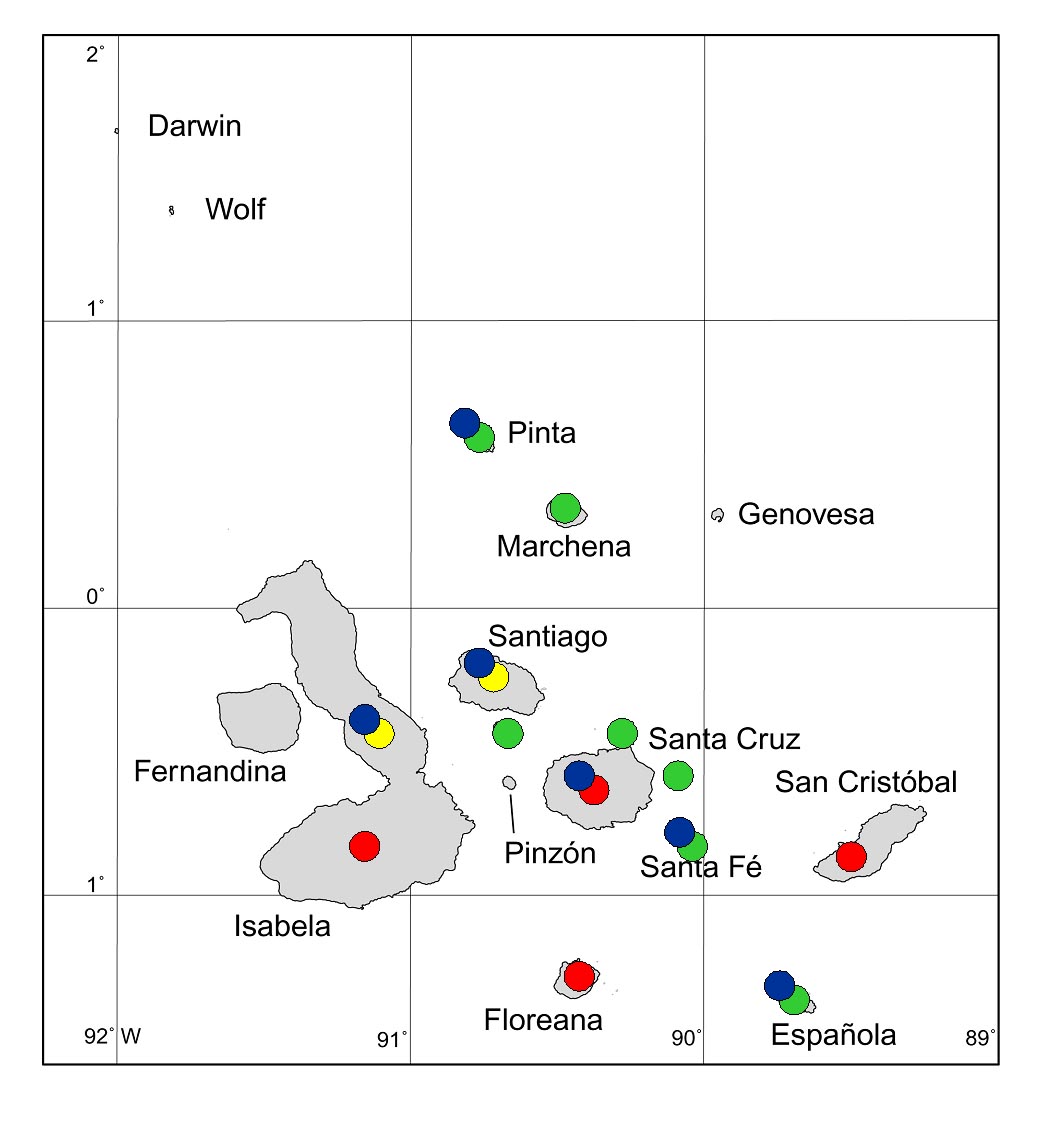
Figure 6. Galapagos islands to which goats have been
introduced and gone wild. Red circles = not yet eradicated; green = already eradicated; yellow = eradication in progress (as at end of 2005); blue = long-term vegetation monitoring projects in place.
Step
3: Monitor the effects of removing or reducing the threat
The importance of monitoring both success of and impacts of control cannot be over-emphasized. This is particularly true in the case of eradication, where we need to be sure that all individuals of the target invasive plant (including viable seeds) or animal have really been removed. The importance of monitoring the impact of control lies in determining whether the effect on native species and communities is the desired one (e.g are native species regenerating in appropriate proportions or are other invasive introduced species replacing them?), and thereby detecting whether any further intervention (Step 4: "restoration") is required: it may not be. Once again, the example of goat eradications on Galapagos illustrates this.
On six of the 12 islands where goats had been introduced, long-term vegetation monitoring projects based on permanent plots and transects have been established, which have so far gathered data for up to almost 40 years (Fig. 6). Goats were eradicated from Santa Fe and Española in the 1970s, and these have since been monitored by Hamann (1979, 2001) and H. Adsersen (unpublished). Hamann and Adsersen have also monitored plots on Pinta (Hamann 1975, 1979, 1993), from which goats were almost eradicated in the 1970s, and then finally eradicated in 2003. Hamann (2001) has also been monitoring plots on Santa Cruz, where goats have been subject to varying levels of control but not eradicated; these plots also demonstrated the changes brought about in Transition Zone forest by Cedrela odorata and Psidium guajava. Many of these island studies were initially established by De Vries (e.g. 1977, De Vries & Calvopiña 1977), who also began monitoring on Santiago, from which goats were not eradicated until 2005. Since the studies of De Vries, successive CDRS botanists have continued monitoring on Santiago, and have established plots in 1995 on Alcedo Volcano (Isabela), from which goats may be eradicated in 2006.
These projects have demonstrated that goat removal is usually followed by rapid regeneration of native vegetation, attaining a structure and species composition closely similar to the pre-goat state within 20 years. The Galapagos flora, as is typical of oceanic islands, consists largely of species with pioneer characteristics and has proved remarkably resilient following removal of introduced species threats. In other words, little further intervention (Step 4 restoration) is usually required, beyond threat removal. We have several lines of evidence that this is so.
For example on Española, photographs taken in 1905-6 by the California Academy of Sciences in various parts of the island demonstrate the state of the vegetation shortly after the introduction of goats, while transect comparisons between Española and its offshore islet Gardner (which never harboured goats) show that the state attained on the main island 20 years after goat eradication is comparable with that of a nearby area unaffected by goats. On Santa Fe, the species-focused studies of Hamann showed that Scalesia helleri (Fig. 7) and Opuntia echios, which had been badly affected by the goats, regenerated more or less rapidly following eradication of the animals.

Figure 7. Scalesia helleri regenerated rapidly
following the eradication of goats from Santa Fe island.
Exclosures also show similar effects on a smaller scale. The only known population of Linum cratericola increased from a low of 13 known plants to more than 400, when most of the population was protected by fences, and goats and feral donkeys were controlled in the surrounding area (Simbaña 2002 and unpublished).
The general conclusion is that most Galapagos vegetation recovers fast once a threat is removed, but does it all? The importance of careful monitoring is demonstrated by cases where individual species have not recovered following goat eradication or exclusion. Three examples include Cyathea weatherbyana on Alcedo, whose last two remnant populations were protected by fences in 1997, and Opuntia megasperma and Lecocarpus lecocarpoides on Española, where goats were eradicated in 1978. Such cases lead us to Step 4 of the restoration process.
Step
4: Further intervention, when necessary to ensure recovery.
In all these cases, the problem is with individual species, rather than failure of a whole community to return to something close to its original state. We therefore require autecological studies to determine what further action needs to be taken to ensure their recovery. Such studies must focus on identifying the additional threat(s) which may be preventing recovery, and identifying the life history stage(s) at which action must be taken to reverse the effect of each threat. Cyathea weatherbyana on Alcedo, and Opuntia megasperma and Lecocarpus lecocarpoides on Española illustrate different stages in this process.
In the case of the Cyathea, it was thought in 1996 that its decline was associated with a process of dramatic vegetation change on the volcano caused by a goat population explosion there in the early 1990s (Cayot & Snell 1996). The planned solution to these changes was to be an attempt to eradicate goats from northern Isabela but, pending a search for funds for such an ambitious project, a temporary measure intended to save the last tree ferns was the fencing of the two remnant groups of plants, which was accomplished in 1997. The two patches were included in the monitoring programme that was begun in 1995. This has shown a continued decline in the species, despite the fence. It has proved difficult to ascertain the reasons for this continued decline. Contributory factors could include occasional breaches of the fence by small groups of goats, which have remained inside the exclosure for periods up to 2-3 months, but these were not closely correlated with population decline of the tree fern. It has not been possible to initiate a detailed study of the remaining plants (or of healthier populations on the other islands), but periodic observations suggest that the plants may be suffering water stress, as they appear to be almost permanently in a semi-wilted state (Fig. 8). This leads to the suspicion that micro-climatic changes as a result of general loss of forest cover in the surrounding areas may be at least partly responsible. Goat eradication finally began in 2003 and at the end of 2005 was virtually complete. Results of monitoring on Santiago Island show that highland vegetation recovery has been astonishingly fast there following goat eradication, with a moderate shrub-tree canopy expected to be reestablished over large areas within 3-4 years. The strategy for Alcedo for the time being is therefore to await the results of the goat eradication there, which is about one year behind that on Santiago, to see if general vegetation recovery promotes regeneration of the tree fern.

Figure 8. Remnant adult Cyathea weatherbyana tree
ferns inside a goat exclosure on Alcedo Volcano, Isabela Island.
Lecocarpus lecocarpoides is still a common species on the four islets in Gardner Bay, Española. On the main island it is known only from a tiny population on the north coast (Fig. 9), which fluctuates between zero and 60 plants, behaving as an annual. Since goat eradication in 1978, this population has not increased and it is not known what restrictions might be preventing an increase. In this case we have no evidence that the species was more common on the main island before the introduction of goats, but suitable habitat seems to be present in several parts of the island. This species has not been the subject of any detailed study so far, nor of any management intervention to try to increase its population. However, a study is planned to examine seed germination and habitat requirements, as a preliminary to an attempt to establish a population at a second site on the main island.
We have more information about Opuntia megasperma, the other Española species which has not increased since goat eradication. This species may be used to illustrate the kind of research that needs to be done to address such a problem.
First, we have better evidence of the former status of Opuntia on Española. The CAS photographs of 1905-6 show that large adults were present in many parts of the island where no Opuntia can be found today, including the eastern extremity, Punta Cevallos. The nearest remaining Opuntia to Punta Cevallos are now some 4 km away, making its return to such sites extremely unlikely in the medium term. Second, we know that, even in areas where adult Opuntia remain, regeneration has been poor or absent (Grant & Grant 1989, Coronel 2002). We have therefore carried out a series of studies to try to understand why.
Our first hypothesis was that seed production or viability might have been reduced, perhaps as a result of a genetic bottleneck, or that seed survival might have been adversely affected by an imbalance between the Opuntia population and those of its seed predators (such as Darwin's finches Geospizinae), resulting in excessive seed predation. Preliminary observation confirmed, however, that adult plants were fruiting normally and that the fruits contained normal numbers of seeds. Neither did seed survival appear to be the problem, since abundant seed could be found on the ground in areas where adult plants still occurred. Further, seed collected from the ground and from fruits was viable. Laboratory experiments gave germination rates of c. 40% with no pre-treatment, while seed collected from the faeces of giant tortoises, after the fruit were fed to them, gave c. 80% germination. Tortoises are natural dispersers of Opuntia seed in Galapagos.
It is still not known whether failure of germination in the field may be a factor contributing to the lack of recovery. We know that germination was high on Española in moderately wet years in the 1970s, although perhaps poorer in exceptionally wet years in the early 1980s (Grant & Grant 1989). It may be that germination requires wetter than average conditions, and Galapagos has received below average rainfall during the years 2002-5. However, no significant regeneration has occurred since the 1970s, during which period a broad range of Galapagos climatic conditions has been represented, from very dry to very wet years. Some factor may therefore have got worse over the intervening period. The presence of some seedlings in limited areas shows that at least some natural germination has occurred in recent years, and further monitoring under different rainfall conditions may help to clarify the role of germination problems.
However, knowing that viable seed production is not the key factor, the question passes to the next life stage: the seedling. The hypothesis here was that seedling survival may have been compromised by lack of water, predation, or other factors. We therefore cultivated seedlings in the laboratory, acclimatized them to field conditions, then planted them out in the field and gave them a variety of post-planting treatments, comprising: given water and protected by small cages; not given water but protected; protected but not given water; and unprotected unwatered (Fig. 10). These experiments showed that water was not a critical factor, but that protection by small cages dramatically improved seedling survival (Coronel 2002). Observations indicate that native animals on Española damage Opuntia seedlings in various ways, either eating them or physically damaging them. Further experiments are being carried out to determine which animals cause important damage, and from which the seedlings require protection. The problem may therefore be, at least in part, an imbalance between the Opuntia (seedlings) population and the populations of its seedling predators. A dramatic increase in the population of one of the animals known to damage young Opuntia, the giant tortoise on Española, is a result of another, highly successful, Galapagos restoration programme. A possible lesson for restoration planners is that this may have contributed to the hypothesized worsening conditions for the seedlings since the 1970s.

Figure 10. Research student Vanessa Coronel with Opuntia megasperma seedlings planted in the field on Española Island.
A final Galapagos example serves to illustrate a case where Step 4 is inevitable: where removal of a threat cannot result in recuperation of a population without further intervention. This is the case where the population is extinct, either locally or completely in the wild. Scalesia atractyloides, endemic to Santiago Island, exists in a number of distinct populations some of which are isolated by unsuitable intervening habitat. Some of the populations show distinctive morphological characteristics, so each is probably genetically valuable. The species was greatly reduced by goats, but most of its populations are expected to recover now that the goats have been almost eliminated from the island. However, at least one population, at Ladilla Bay (Fig. 11ab), became extinct in the 1980s or 1990s, but not before seed from this population had been taken into cultivation in Copenhagen Botanic Garden. After the goats have been confirmed eradicated from Santiago, we intend to use seed from Copenhagen in an attempt to re-establish this population.
Conclusions 
Restoration Messages from the Islands
"Restoration" may be seen in its more restricted sense as a part of a conservation strategy, or the term may be used to define that strategy. In this broader sense, restoration includes three main steps, understanding the threat, removing or reducing the threat, and observing the result. If the required result is not obtained, then further intervention (restoration in its more restricted sense) may be required.
However, examples from Galapagos show that in the case of an oceanic archipelago, with a flora with pioneer characteristics, the vegetation is often sufficiently resilient that this final or additional step is not required. Where the threat factor is an introduced species, simply removing that species may be enough to ensure full recovery of the native vegetation, especially on islands where other introduced species are not available to replace the first.
On the other hand, when further intervention is required, this comprises in effect a return to the original three steps: identifying and understanding additional threats, removing or reducing those, and watching what happens. Further, in contrast to the first round of the three initial steps, where this final step (or new cycle of steps 1-3) is required, it usually requires action directed at an individual threatened species, whereas the first round usually deals with a threat that affects an entire vegetation community. The kinds of ecological studies and monitoring required thus differ completely in a second round of "restoration", where a species focus is appropriate, from those required in the first round, where the focus is on vegetation communities.
Acknowledgements 
I would like to express my profound gratitude to my colleagues and predecessors in the CDRS Botany Department for their hard work over the years on the projects mentioned in this article, and for the use of their photographs. Unacknowledged photos are my own. This is Contribution 1030 of the Charles Darwin Research Station.
References 
Baker J.K. & D.W. Reeser. 1972. Goat management problems, Hawaii Volcanoes National Park. Natural Resources Reports 2, US Dept of the Interior National Park Service.
Bensted-Smith, R. (ed.) 2002 (online); in press (2006) as book. A Biodiversity Vision for the Galapagos Islands. Charles Darwin Foundation and WWF, Puerto Ayora. Online at: http://www.darwinfoundation.org/downloads/bio_vision_galapagos_eng.pdf
Breckon G.J. 2000. Revision of the flora of Desecheo Island; Puerto Rico. Caribbean Journal of Science 36: 177-209.
Buddenhagen, C. & P. Yánez. 2005. The cost of Quinine Cinchona pubescens control on Santa Cruz Island, Galapagos. Galapagos Research 63: 32-36.
Cayot, L. & H.M. Snell. 1996. Goats damage Volcán Alcedo, Isabela. Noticias de Galápagos 56: 3.
Coblentz, B.E. 1978. The effects of feral goats (Capra hircus) on island ecosystems. Biological Conservation 13: 279-286.
Coronel, V. 2002. Distribución y re-establecimiento de Opuntia megasperma var. orientalis Howell. (Cactaceae) en Punta Cevallos, Isla Española - Galápagos. Tesis de grado, Universidad del Azuay, Cuenca, Ecuador.
Courchamp F., J.-L. Chapuis & M. Pascal. 2003. Mammal invaders on islands: impact, control and control impact. Biological Reviews 78: 347-383.
De Vries, T. 1977 Como la caza de chivos afecta la vegetación en las islas Santa Fé y Pinta, Galápagos. Revista de la Universidad Católica 16: 171-181.
De Vries, T. & Calvopiña, L.H. 1977. Papel de los chivos en los cambios de la vegetación de la isla San Salvador, Galápagos. Revista de la Universidad Católica 16: 145-169.
Gould, M.S. & I.R. Swingland. 1980. The tortoise and the goat: interactions on Aldabra island. Biological Conservation 17: 267-279.
Grant, B.R. & P.R. Grant 1989. The slow recovery of Opuntia megasperma on Española. Noticias de Galápagos 48: 13-15.
Hamann, O. 1975. Vegetational changes in the Galapagos Islands during the period 1966-73. Biological Conservation 7: 37-59.
Hamann, O. 1979. Regeneration of vegetation on Santa Fé and Pinta Islands, Galápagos, after the eradication of goats. Biological Conservation 15: 215 - 236.
Hamann, O. 1993. On vegetation recovery, goats and giant tortoises on Pinta Island, Galapagos, Ecuador. Biodiversity and Conservation 2: 138-151.
Hamann, O. 2001. Demographic studies of three indigenous stand-forming plant taxa (Scalesia, Opuntia, and Bursera) in the Galapagos Islands, Ecuador. Biodiversity and Conservation 10: 223-250.
Jäger, H. 1999. Impact of the introduced tree Cinchona pubescens Vahl on the native flora of the highlands of Santa Cruz Island (Galapagos Islands). Diplomarbeit, University of Oldenburg, Germany.
Lever, C. 1994. Naturalized Animals. Poyser, London.
Loope, L.L. & P.G. Scowcroft. 1985. Vegetation response within exclosures in Hawai'i: a review. Pp. 377-431 in: C.P. Stone & J.M. Scott (eds) Hawai'i's Terrestrial Ecosystems: Preservation and Management. University of Hawaii, Manoa.
Mauchamp, A., I. Aldaz, E. Ortiz & H. Valdebenito. 1998. Threatened species, a reevaluation of the status of eight endemic plants of the Galápagos. Biodiversity and Conservation 7: 97-107.
North, S.G. & D.J. Bullock. 1986. Changes in the vegetation and populations of introduced mammals of Round Island and Gunner's Quoin, Mauritius. Biological Conservation 37: 99-117.
PRONAREG, ORSTOM & INGALA 1987. Islas Galápagos. Mapa de formaciones vegetales. PRONAREG, ORSTOM, INGALA, Quito.
Rentería, J.L. 2002. Ecología y manejo de la Cascarilla (Cinchona pubescens Vahl), en Santa Cruz, Galápagos. Tesis de Grado, Universidad Nacional de Loja, Ecuador.
Richardson, D.M., P. Pyek, M. Rejmánek, M.G. Barbour, F.D. Panetta & C.J. West. 2000. Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions 6: 93-107.
Simbaña, W.A. 2002. Ecología y biología reproductiva de Scalesia atractyloides Arnott, Scalesia stewartii Riley (Asteraceae) y Linum cratericola Eliasson (Linaceae), especies endémicas amenazadas en Galápagos. Tesis de grado, Universidad Central del Ecuador, Quito.
Snell, H.L., A. Tye, C.E. Causton & R. Bensted-Smith. 2002 (online); in press (2006) as book. The status of and threats to terrestrial biodiversity. Chapter 5 in A Biodiversity Vision for the Galapagos Islands. Charles Darwin Foundation and WWF, Puerto Ayora. Online at: http://www.darwinfoundation.org/downloads/bio_vision_galapagos_eng.pdf
Tye A. 2002. Revision of the threat status of the endemic flora of Galapagos. Galapagos Report 2001-2002. pp 116-122. WWF - Fundación Natura, Quito.
Wiggins, I.L. & D.M. Porter. 1971. Flora of the Galápagos Islands. Stanford University Press. Stanford, USA. Fig 5 both photos M. Gardener, Fig 11 right photo P. Jaramillo