Introduction 
Mangrove forests, dominated by estuarine trees serve as ecotones between land and sea and elements from both are stratified horizontally and vertically, between the forest canopy and subsurface soil (Rao & Deshmukh, 1994). Mangrove has been defined as "any woody, tropical halophyte that is an obligate inhabitant of 'mangal' (wetland community) (Tomlinson ,1986). The word mangrove has traditionally been used to describe either the total community or the individual tree/ bushes, growing in the clayey, silty, inter-tidal coastal zones, deltaic and estuarine coasts and backwaters/ sheltered regions, in the tropical/subtropical belts of the world (Nayak & Bahuguna, 2001). Mangroves can often survive non- saline habitats (Cintron & Schaeffer-Novelli, 1983; Walsh, 1974). However, according to Lugo (1980), a saline environment is required for stable mangrove ecosystems. About 54-70 species (including hybrids) in 20-27 genera and 16-19 families fit comfortably into this broad category (Tomlinson, 1986; Cronquist, 1981; Duke, 1992). Mangrove areas have wide range of families, including ferns, grasses, sedges, palms and legumes.Mangroves grow throughout the tropics wherever the average monthly minimum temperature is 200 C (Chapman, 1976)and are believed to be limited in their subtropical distribution by lack of low temperature resistance (Dodd et al. 1995). Between 250 N and 250 S, mangroves colonize almost 75 % of the coastline (Day et al., 1987) although they only represent 1 % (100000 km2) of the area of tropical forest are quite productive (350 to 500 gram C m-2 yr-1) (Mann, 1982). Mangroves may show strong, weak or no spatial zonation (Tomlinson, 1986; Ellison et. al., 2000), although the abundance of individual species may follow the gradient of salinity (Helalsiddiqui, 1999). Mangroves prefer a salinity range of 5- 30 parts per thousand.
The ecological importance of these ecosystems for maintaining marine life was stressed by Upadhyay et al. (2002); Fromard et al. (1998); Odum & Heald (1975). Studies have demonstrated their role in supplying organic material to coastal marine ecosystems (Odum & Heald, 1972; Lugo et al. 1980; Boto & Bunt, 1981; Rojas-Beltran, 1986; Hutching & Saengar, 1987). Mangrove ecosystems are being studied with more interest worldwide because of their economic importance in support of commercial fisheries alone (Cintron et al. 1980). Uses and values of mangroves are many and varied. For example, they provide habitat as well as spawning and nursery ground for various marine species (fish, shellfish, crustaceans etc), enrich the near-shore environment, act as windbreakers and protects the shoreline from storms, stabilize the shoreline, and decrease coastal erosion (Nayak & Bahuguna, 2001).
Out of 4,87,100 ha. of mangrove wetlands in India, nearly 56. 7 % (2,75,800 ha.) is present along the east coast, 23. 5 % (1,14,700 ha.) along the west coast, and the remaining 19.8 % (96,600 ha.) is found in the Andaman and Nicobar islands (FSI,1999),. Mangroves in the densely populated East Coast of India have been degraded for decades and are still continuing to be degraded due to loss of biomass, species composition simplification mainly due to overgrazing, fuel wood extraction and conversions (Blasco & Aizpuru, 2002).
Mangroves are spread over an area of 214 sq. km (FSI, 1999) in Orissa. The assessment has indicated an increase of 20 sq. Km (FSI, 1997, 1999) in Bhadrak and Kendrapara districts. Although the overall assessment shows an increase, several areas have shown marked decrease in quality and quantity of the vegetation cover. Causes for degradation of mangroves in Orissa are, shoreline changes, settlements, conversion for agriculture and aqua culture (Upadhyay et al. 2002). Recent researches carried out on biosystematics of mangrove phanerogams (Dodd et. al. 1995; Duke, 1995; Tomlinson, 1986); on biogeography (Saengar, 1996); ecology (Snedaker, 1995) and distribution (Spalding et al. 1997) can be considered as being of direct interest to the knowledge of the mangroves of India (Blasco & Aizpuru, 1997). Taxonomical works on mangroves have been done by Banerjee et al. (1989), Banerjee (1984, 1987); Banerjee & Rao (1990); Choudhury (1984, 1990); Choudhury et al. (1991, 1995); Majumdar & Banerjee (1985); Mishra & Panigrahi (1987).The present paper highlights ecological structures of mangroves ecosystem of Orissa coast based on phytosociological studies.
The Study Site
The state of Orissa has a geographical area of 155707 sq. km with an actual forest cover of 47107 sq. km. (30.3 %). Area under Mangrove forests is 195 sq. km which comes to 0.125 % of geographical area and 0.414 % of actual forest cover (Daniels & Acharjyo, 1997). The study site located at 200 4'- 200 8' N Latitude and 860 45'- 870 5' E Longitude, in the north-eastern coastal plain of Kendrapara district in Orissa is in Bhitarkanika sanctuary. Total area of sanctuary is 672 Sq. km of which mangrove forests constitute 130 sq. km. This area receives water from three rivers, known to be rich in species diversity and trees are dense and tall like those of Sunderbans (Selvam, 2003). Four forest blocks in the Bhitarkanika wildlife sanctuary were selected for carrying out vegetation survey. The area of Bhitarkanika forest block is 1712 ha., Dangmal 636 ha., Kakranasi 310 ha., and Thakurdia 272 ha. (Chadha & Kar, 1999). Bhitarkanika and Dangmal Bocks constitute the core area. These sites experience tide of semi diurnal type. The mean sea level in the region is about 1. 66 meters. The Bhitarkanika sanctuary is bounded by river Dhamra in the north, the river Hansua to the west and Bay of Bengal on the eastern and southern sides. The sanctuary encompasses 35 km sea coast known as 'Gahirmatha Coast' from Dhamra mouth to Barunei, the mouth of river Hansua. The area has about 200 km. of water body inside the sanctuaryand falls in the deltaic region of the river Brahmani, Baitarani, and their tributaries. The estuarine rivers- Brahmani, Baitarani, Kharasrota, Dhamra, Pathasala, Maipura, Hansua, and Hansina during their course flow into the Bay of Bengal are further criss crossed by numerous creeks, channels, and nallahs, thus providing the peculiar ecological niche for the growth, development of rich and varied mangrove life forms, both flora and fauna along with their associates. There are many villages within the sanctuary as well as surrounding it. The population in these villages has been growing very fast. Part of the population rise is because of the heavy influx of refugees from East Bengal and West Bengal and habitations are reported to have been started by clearing mangrove forests. A total of 81 villages are adjacent to the mangrove forests. The population increase is attributed as one of the reasons for decreasing mangrove of the area.
Climate
The region comes under the tropical monsoon climate with three pronounced seasons: winter (October to January), summer (February to May) and rainy (June to September). The maximum temperature is recorded in the month of April and May and the minimum temperature in winter during the month of January. The relative humidity ranges from 70% to 84% through out the year. Wind speed from March to June is over 20 km. per hour, and the predominant wind direction is from south and south-west. Rainfall is around 1642.34 cm per annum and maximum rainfall is received between June and October. The most important weather phenomenon is the prevalence of tropical cyclones. The mean track of the cyclone passes over this region (Singh & Panda, 1999). Rainfall conditions decide the sequence of mangrove distribution in the different zones in the tidal region. A successive tidal flood inundates the land surface and the subsequent exposure of the soil substratum evaporates the water. This result in thick salt crust on the soil surface and these salt crusts inhibit or limit the regeneration and growth of the mangroves. Frequent rainwater flushing helps in washing off the surface and leaching down the salt particles and makes the land suitable for growth of mangroves. Tidal amplitude in the Baunsagada River ranges from 1.5 to 2.5 meters in summer months to 3 to 5 meters during monsoon months. In the Bhitarkanika River, and especially in creeks such as Khola (which receives tidal water from both ends) tidal amplitude reaches 3- 4 meters in summer months to 5-6 meters during rainy season.
Soils and Geology
The soil sediments are divided into two categories, indicating recent or sub-recent forms named as 'newer alluvium' and Pleistocene forms named as 'older alluvium' (GSI, 1974). The recent sediments are represented by sand, silt, and clay with assorted boulders and pebbles. These are dark and loosely compacted with high moisture content. The Pleistocene deposits comprise of clay, sand, silt, and 'kankar', with locally cemented pebbles and gravels. These are reddish brown due to high degree of oxidation (Banerjee & Rao, 1990).
Methods 
Phytosociological Analysis in four forest blocks was carried out by quadrat method following Misra (1968), Kershaw (1973), Cintron and Schaefer-Novelli (1984) and Snedaker & Snedaker (1984). Thirty quadrats of 10m X 10 m size were laid out at each site. Each site was divided into 6 segments of 1 km each along tidal line from the riverbank. A line transect was laid towards landward side from the water line. In each segment, 6 quadrats of 10 m X 10 m size were laid at 0, 50, 100, 150, 200 and 250 meter interval towards the land ward side for phytosociological analysis. 120 quadrats were laid in four forest blocks to study forest structure (trees). On the basis of data obtained from quadrat samples, the structural parameters like frequency, abundance, density, basal area, and IVI were calculated (Tables 1a-d).
Results and Discussion 
Vegetation Analysis
The
Bhitarkanika forest block contains highest number of tree species
followed by Dangmal, Kakranasi and Thakurdia blocks. Bhitarkanika and
Dangmal are part of core area of the Bhitarkanika wildlife sanctuary.
Availability of fresh water through Bhitarkanika (Maipura river) and
Brahmani rivers and saline water from sea in core area help wide
range of niches for different species to occur and, thus, species
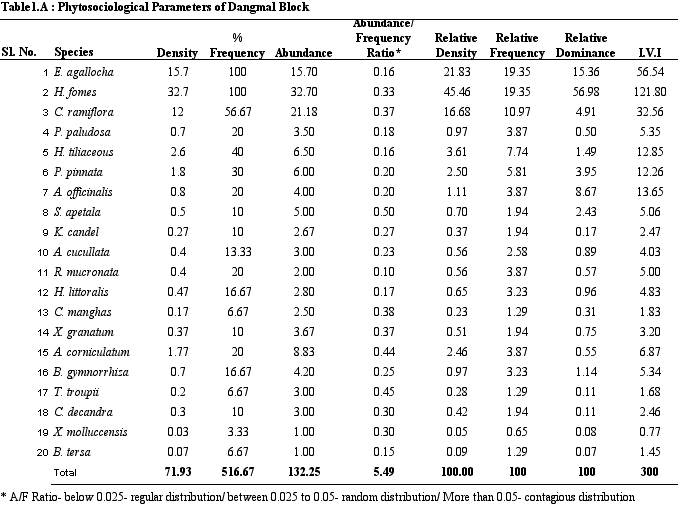
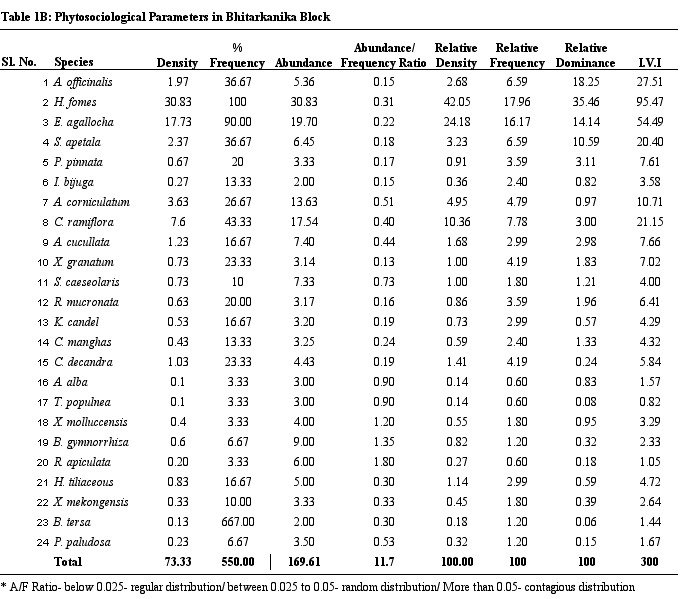
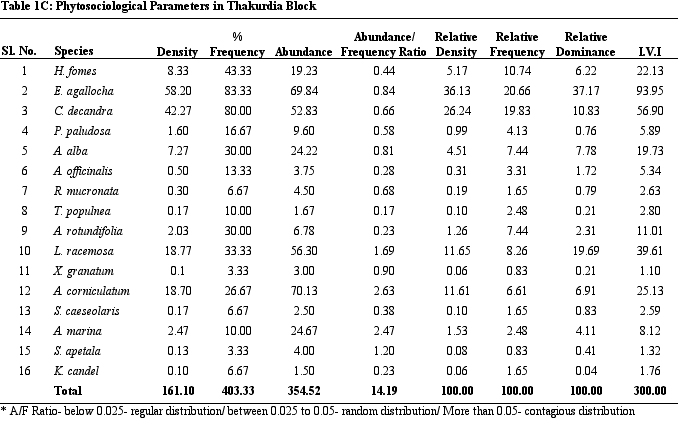
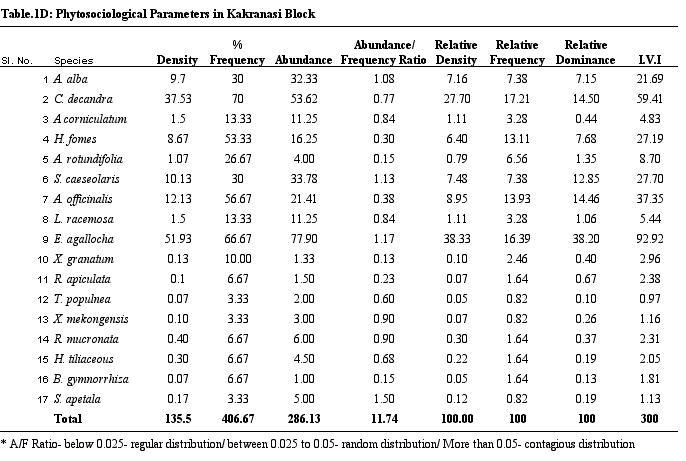
diversity is the highest. Table 1 provides details on structural
parameters of vegetation of study sites. H. fomes and E.
agallocha exhibited greater density, frequency and IVI values
across all sites. The species with lower density and IVI are
different from one site to the other. All the species show contagious
distribution. A/F ratio range in Dangmal block is proportionately
less wide compared to other blocks. According to Odum (1971)
contagious distribution is commonest in nature, random distribution
is found only in very uniform environment and regular distribution
occurs where severe competition exists between individuals.
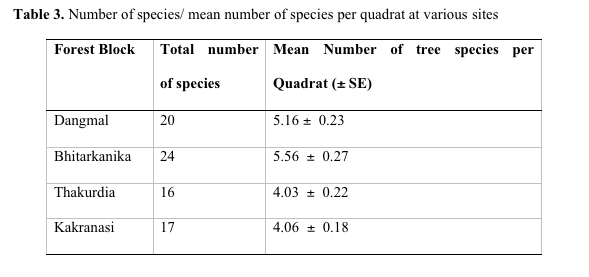
From list of species encountered through quadrat surveys of trees and seedlings (Table 2), it is observed that the family Rhizophoraceae and Meliaceae represented maximum number of species followed by Avicenniaceae. Bhitarkanika is the most species rich site with 24 species and Thakurdia has the lowest species number with 16 species (Table 3). Bhitarkanika has the highest mean species value per quadrat (5.56 species per quadrat). The average value for all the forest blocks is 4.69 species per quadrat. Ellison (2002) established a correlation between latitude and longitude and species richness and observed that the species richness is higher (> 30 to 55 species) between 0 and 200 N lat and at 70 and 1350 E long. Species richness is highest in the Indo West Pacific and declines relatively smoothly from 1000 E which is the longitude of peak species richness (Ellison et al. 1999).
Table 2. Mangrove and associated species in the study
area
| Sl No | Species encountered through quadrat survey | Species encountered through seedling survey | Other species encountered during survey |
|
|
Dicotyledons |
|
|
| 1 | Acanthaceae |
|
|
|
|
|
Acanthus ilicifolius |
|
| 2 | Aizoaceae |
|
|
|
|
|
Sesuvium portulacastrum |
|
| 3 | Apocynaceae |
|
|
|
|
Cerbera manghas | Cerbera manghas |
|
| 4 | Avicenniaceae |
|
|
|
|
Avicennia alba |
|
|
|
|
Avicennia officinalis | Avicennia officinalis |
|
|
|
Avicennia marina |
|
|
| 5 | Caesalpiniaceae |
|
|
|
|
|
Caesalpinia crista |
|
|
|
Cynometra ramiflora | Cynometra ramiflora |
|
|
|
Intsia bijuga | Intsia bijuga |
|
| 6 | Chenopodiaceae |
|
|
|
|
|
|
Salicornia brachiata |
|
|
|
S. maritima | |
| 7 | Combretaceae |
|
|
|
|
Lumnitzera racemosa |
|
|
| 8 | Euphorbiaceae |
|
|
|
|
Excoecaria agallocha | Excoecaria agallocha |
|
| 9 | Malvaceae |
|
|
|
|
Thespesia populnea | Thespesia populnea |
|
|
|
Hibiscus tiliaceous | Hibiscus tiliaceous |
|
| 10 | Meliaceae |
|
|
|
|
Amoora cucullata |
|
|
|
|
Xylocarpus granatum | Xylocarpus granatum |
|
|
|
Xylocarpus mekongensis | Xylocarpus mekongensis |
|
|
|
Xylocarpus molluccensis | Xylocarpus molluccensis |
|
| 11 |
Myrsinaceae
|
|
|
|
|
Aegiceras corniculatum | Aegiceras corniculatum |
|
| 12 | Papilonaceae |
|
|
|
|
|
Dalbergia spinosa |
|
|
|
Pongamia pinnata | Pongamia pinnata |
|
| 13 | Peripocaceae |
|
|
|
|
|
|
Finlaysonia obovata |
| 14 | Plumbaginaceae |
|
|
|
|
Aegialitis rotundifolia |
|
|
| 15 | Rhizophoraceae |
|
|
|
|
Bruguiera gymnorrhiza | Bruguiera gymnorrhiza |
|
|
|
Ceriops decandra | Ceriops decandra |
|
|
|
Kandelia candel | Kandelia candel |
|
|
|
Rhizophora apiculata | Rhizophora apiculata |
|
| 16 | Rutaceae |
|
|
|
|
|
|
Merope angulata |
| 17 | Salvadoraceae |
|
|
|
|
|
|
Salvadora persica |
| 18 |
Sonneratiaceae
|
|
|
|
|
Sonneratia apetala |
Sonneratia apetala |
|
|
|
Sonneratia caeseolaris | Sonneratia caeseolaris |
|
| 19 | Sterculiaceae |
|
|
|
|
Heritiera fomes | Heritiera fomes |
|
|
|
Heritiera littoralis | Heritiera littoralis |
|
| 20 | Tamaricaceae |
|
|
|
|
Tamarix troupii | Tamarix troupii |
|
| 21 | Tiliaceae |
|
|
|
|
Brownlowia tersa | Brownlowia tersa |
|
| 22 | Verbenaceae |
|
|
|
|
|
|
Clerodendrum inerme
|
|
|
Monocotyledons |
|
|
| 23 | Arecaceae |
|
|
|
|
Phoenix paludosa | Phoenix paludosa |
|
| 24 | Flagellariaceae |
|
|
|
|
|
|
Flagellaria indica |
| 25 | Poaceae |
|
|
|
|
|
Myriostachya wighitiana |
|
|
|
|
Porteresia coarctata |
|
| 26 | Polypodiaceae |
|
|
|
|
(fern) | Acrostichum aurum |
|
A total of 22 families of Dicotyledons and 4 families of Monocotyledons were represented across all sites in Bhitarkanika Mangrove ecosystem. A total of 43 species of mangrove and associated plants belonging to 32 genera were recorded from 26 families of Angiosperms. The flora is extremely diverse in the estuarine regions of Bhitarkanika, (Banerjee & Rao 1985, 1990).Abundance of phanerogams is presumably higher than that of the Sunderbans Gangetic delta. Though the factors influencing biodiversity and floristic richness in each deltaic region is not fully understood (Duke et al. 1998), the assumption is that the propagules originated in the Sunderbans are water buoyant and dispersed to the nearest deltaic area which is the mouth of the Mahanadi river. This could explain the high degrees of relationship between the flora of the Gangetic and the Mahanadi deltas (Blasco & Aizpuru, 2002). H. fomes, Sonneratia. griffithii and Aegialitis rotundifolia Roxb. are endemic to the coastal part of South Asia (Blasco et al. 2001) and later two species are not recorded during the study.
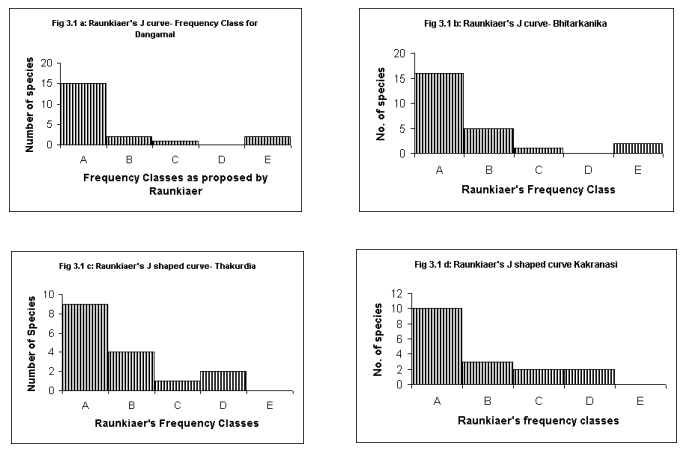
Raunkiaer's Frequency Class Distribution
Raunkiaer's Law of Frequency (in graphical form referred to as Raunkiaer's J shaped distribution curves) was studied (Raunkiaer, 1934). The law (also known as the law of homogeneity) was expressed as A> B > C ≤≥D E, wherein, A to E are frequency classes suggested by Raunkiaer's from 0 to 100. According to Kershaw (1973), "the increase in class E reflects the theoretical infinite range of density and contrasts with the more strictly defined limits for classes A, B, C, and D. This E class has a density range greatly exceeding frequency classes A to D. Accordingly many more species fall into this class, despite the general tendency for 'common' species to be relatively few in number in a community"(Fig.1 ).
Species Diversity
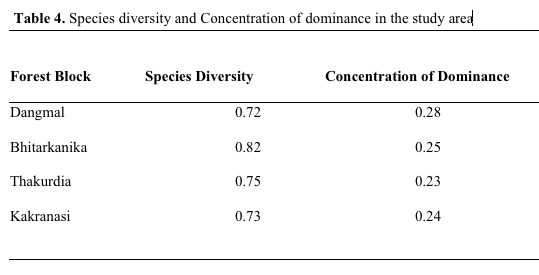
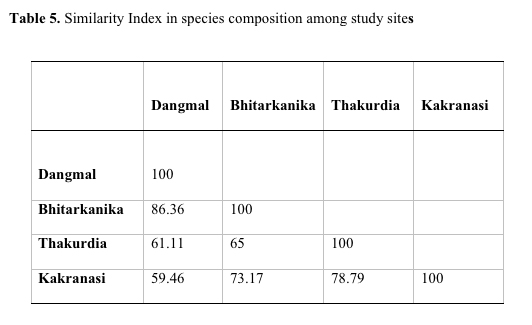
The species diversity depends upon adaptation of species and increases with stability of community (Singh et al, 1994). Species diversity was 0.72, 0.82, 0.75, 0.73, respectively, in Dangmal, Bhitarkanika, Thakurdia, and Kakranasi blocks. The above data indicate that Bhitarkanika site is highly diverse and Dangmal the least. The Concentration of Dominance was 0.28, 0.25, 0.23, 0.24, respectively, in Dangmal, Bhitarkanika, Thakurdia, and Kakranasi indicating the dominance is more pronounced in Dangmal block (Table 4). The Dangmal and Kakranasi blocks exhibited least similarity in species composition (59.46 %) with each other followed by Thakurdia and Bhitarkanika (65 %), Thakurdia and Kakranasi (78.79 %), and Bhitarkanika and Dangmal blocks (86.36%) (Table 5). The latter two sites are adjacent to each other and thereby there is a great deal of species mix. In the Eastern hemisphere number of mangrove species reported by Tomlinson (1986) and Duke (1992) are 58 compared to only 12 in Western hemisphere. High mangrove diversity in South East Asian region is because it has been the center of origin of mangrove speciation. There is presence of adjacent diverse terrestrial flora which has enabled diversity to increase and prevented extinctions (Ricklefs & Latham, 1993). Duke et al. (1998) found the Indo-Malaysia region with most mangrove species number with 48 species.
The species diversity is higher in the India mangrove ecosystems compared to that of Latin America and Africa. Large physical forces in tidewater, salinity level, and lack of stable substratum are some of the natural factors that affect the species diversity (Pathway et al. 2002).Studies on the changes in the species composition for Bhitarkanika are not available like other mangrove areas on the east coast i.e., Sunderbans, Pichavaram and Muthupet, Guava, and Andaman & Nicobar Islands (Kannupandi & Kannan, 1998; Caratini et al., 1973; Mathuda, 1959; Azariah et al. 1992). H. fomes is known to require low soil and water salinities. When the salinity increases, the species becomes stunted, rare, and ultimately disappears. It is known to be 'top dying' (trees shedding their leaves due to stress and could be dying) in parts of Bangladesh(Siddiqi, 1998) and Sunderban because of the increase in dry season demand for freshwater, damming of rivers and apparent downstream effects of increase in soil salinities (Blasco et al. 2001). Therefore, this species is a leading dominant in the mangroves of Bhitarkanika, and thereby confirms to the availability of good ecological conditions that harbours it well. However, caution has to be exercised to see that the preconditions that are now suitable continue to be so. In the mangrove areas of Myanmar H. fomes was available in plenty between the mouth of Mayu and Lamu city about 50 years back and has been completely depleted due to high salinity stress (Blasco, et al. 2001). Others have also reported about die back of Heritiera fomes due to adverse increase in soil salinity (Christensen & Snedaker, 1984; Chaffey et al. 1985).
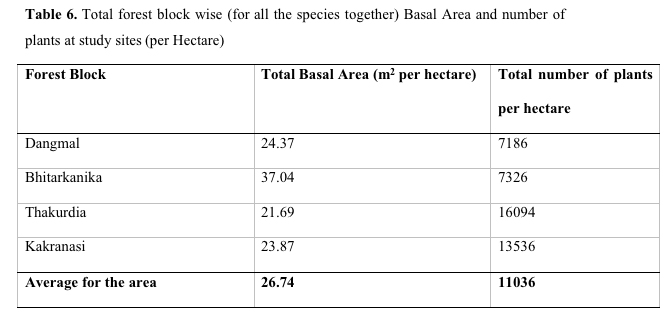
Several authors have worked on phytosociological parameters of Tropical Mangroves. In French Guiana forests Fromard et al. (1998) observed that in mature coastal and adult riverine mangrove sites Avicennia exhibited the highest value of IVI (144 - 181) followed by Rhizophora species. These mangrove types are more frequent in Guiana and are homogenous and dominated by A. germinatus. The mangroves on sea fronts generally have high basal area (24.6 - 33.6 m2 ha-1). The riverine mangrove ecosystems are more diversified and mixed type and richer in species having tree density. However, the species, H. fomes and E. agallocha exhibited dominance with high value of IVI followed by A. officinalis in the mangroves of Orissa coast at Bhitarkanika.
References 
Azariah, J, V. Selvam & S. Gunasekaran. 1992. Impact of past management practices on the present status of the Muthupet mangrove ecosystem. Hydrobiologia 247: 253- 259.
Banerjee, L.K. 1984. Vegetation of Bhitarkanika sanctuary in Cuttack, Orissa. India, Journal of Economic & Taxonomic Botany 5: 1065- 1079.
Banerjee, L.K. & T.A. Rao. 1985. Mangals of Mahanadi delta, Cuttack district, Orissa state, India, in: Marine Plants, paper presented at the All India Symposium on Marine Plants, Goa.
Banerjee, L.K., A.R.K. Sastry & M.P.Nayar. 1989. Mangroves in India-identification manual. Botanical Survey of India, 113 pages.
Banerjee, L.K. & T.A. Rao. 1990. Mangroves of Orissa Coast and their ecology. Bishen Singh Mahendra Pal Singh, Dehra Dun, India, 118 pages.
Banerjee, L.K., 1987. Ecological studies on the mangals of Mahanadi estuarine delta, Orissa. Tropical Ecology 82: 117- 125.
Blasco, F. & M. Aizpuru. 1997. Classification and evolution of the mangroves of India. Tropical Ecology 38: 357- 374.
Blasco, F, M. Aizpuru & C. Gers. 2001. Depletion of the mangroves of continental Asia. Wetlands Ecology and Management 9: 245- 256.
Blasco, F & M. Aizpuru. 2002. Mangroves along the coastal stretch of Bay of Bengal: present status. Indian Journal of Marine Science 31: 9- 20.
Boto, K.G. & J.S. Bunt. 1981. Tidal export of particulate organic matter from a southern Australian mangrove system. Estuarine Coastal Shelf Sci. pp- 247- 255.
Caratini, C., F. Blasco & G. Thankaimoni. 1973. Pollen Spores 15: 281- 292.
Chadha, S. & C.S. Kar. 1999. Bhitarkanika: Myth and Reality. Natraj Publishers, Dehradun, 368
Chaffey, D.R., F.R. Miller & J.H. Sandom. 1985. A forest inventory of the Sunderbans, Bangladesh. Main Report, ODA, Project Report, 140.
Chapman, V.J. 1976. Mangrove Vegetation. J. Cramer, Vaduz, Liechtenstein.
Choudhury, B P. 1984. A glimpse into the vegetation of Bhitarkanika wildlife sanctuary in the state of Orissa. India. Bot. Reptr. 3: 121- 124.
Choudhury, B.P. 1990. Bhitarkanika: mangrove swamps. Journal of Environmental Science 3: 1- 16.
Choudhury, B.P., A.K. Biswal & H.N. Subudhi. 1991. Mangroves of Orissa and aspects of their conservation. Rheedea 1: 62- 67
Choudhury, B.P., A.K. Biswal & S.P. Rath. 1995. Biodiversity in the Bhitarkanika wildlife sanctuary in the state of Orissa. In: (R.C. Mohanty (ed.). Environment: Change and management . New Delhi, pp- 53- 60.
Christensen, B. & S.C. Snedaker. 1984. Integrated development of the Sunderbans: ecological aspects of the Sunderbans. FAO: Field Document No 3, FO: TCP/BGD/2309 (Mf), Rome.
Cintron, G.Y. & Y. Schaeffer-Novelli. 1983. Introduction a la ecologia del manglar, Montevideo, UNESCO, 99 pages.
Cintron, G. & Y.S. Novelli. 1984. Methods for studying mangrove structure. In: Samuel C. Snedaker & J.G. Snedaker- (eds.). The mangrove ecosystem: research methods . UNESCO, 251 pages.
Cronquist, S. 1981. An integrated system of classification of flowering plants. Columbia University Press, New York.
Day, J.W., W. Conner, F. Ley-Lou, R.H. Day & A.M. Navarro. 1987. The productivity and composition of mangrove forests, Laguna de Terminos, Mexico. Aquatic Botany 27: 267- 2844.
Dodd, R.S., F. Fromard, Z.A. Rafii & F. Blasco. 1995. Biodiversity among West African Rhizophora, Foliar Wax Chemistry. Biochemical Systematics and Ecology 23: 859- 868.
Duke, N.C.. 1992. Mangrove floristics and biogeography. In: A.I. Robertson & D.M. Alongi (eds). Tropical Mangrove Ecosystems. American Geophysical Union, Washington DC, pp- 63- 100.
Duke, N.C. 1995. Genetic diversity, distributional barriers, and rafting continents- more thoughts on the evolution of mangroves. Hydrobiologia 295: 167- 181.
Duke, N.C., M.C. Ball & J.C. Ellison. 1998. Factors influencing biodiversity and distributional gradients in mangroves. Global Ecol. Biogeogr. 7: 27- 47.
Ellison, A.M., E.J. Farnsworth & R.E. Merkt. 1999. Origins of mangrove ecosystems and the mangrove biodiversity anomaly. Global Ecol Biogeogr. 8: 95- 115.
Ellison, A.M., B.B. Mukherjee & A. Karim. 2000. Testing patterns of zonation in mangroves: scale dependence and environmental correlates in the Sunderbans of Bangladesh. Journal of Ecology 88: 813- 824.
Ellison, A.M. 2002. Macroecology of mangroves: large scale patterns and processes in tropical coastal forests. Trees 16: 181- 194.
Forest Survey of India. 1999. State of Forest Report- 1999. Dehra Dun
Fromard, F., H. Puig, E. Mougin, G. Marty, J.L. Betoulle & L. Cadamuro. 1998. Structure, above- ground biomass and dynamics of mangrove ecosystems: new data from French Guiana, Oecologia, 115: 39- 53.
GSI. 1974. Geological Survey of India Miscellaneous publication no. 30.
Helalsiddiqui, A.S.M. 1999. Status of the major mangrove species in the Sunderbans of Bangladesh. Indian Journal of Forestry22 : 197- 202.
Hutchings, P. & P. Saengar. 1987. Ecology of mangroves. University of Queensland Press, St. Lucia.
Kannupandi, T. & R. Kannan. 1998. Anthology of Indian Mangroves. ENVIS Center, Annamalai University, Chidambaram, pp- 25- 29.
Kershaw, K.A. 1973. Quantitative and Dynamic Plant ecology (second edition), Edward Arnold, London.
Lugo, Ariel E. 1980. Mangrove ecosystems: successional or steady state? Biotropica 12: 65- 72.
Lugo, A.E., R.R. Twilley & C. Patterson-Zucca. 1980. The role of black mangrove forests in the productivity of coastal ecosystems in south Florida. U. S. Environment Protection Agency, Corvallis, Oregon.
Majumdar, N.C. & L.K. Banerjee. 1985. A new species of Heritiera (Sterculiaceae) from Orissa.Bulletin of Botanical Survey of India 27: 150- 151.
Mann, K.H. 1982. Ecology of coastal waters. University of California Press, Berkeley.
Mathuda, G.S. 1959. Proceedings of the mangrove symposium. Ministry of Food and Agriculture, Kolkata.
Mishra, S.C. & G. Panigrahi. 1987. Studies on the mangrove flora of Orissa with particular reference to Rhizophoraceae. R. Br. J. Econ. Tax. Bot.. 11: 121- 132.
Misra, R. 1968. Ecology Work Book. Oxford and IBH Publishing Co./ New Delhi.
Nayak, S. & A. Bahuguna. 2001. Application of remote sensing data to monitor mangroves and other coastal vegetation of India. Indian Journal of Marine Sciences 30: 195- 213.
Odum, E.P. 1971. Fundamentals of ecology. W. B. Saunders, Philadelphia.
Odum, W. & E.J. Heald. 1972. Trophic analysis of an estuarine mangrove community. Bull. Marine Sci. 22: 671- 738.
Odum, W.E. & E.J. Heald, 1975. Mangrove forests and aquatic productivity In: A.D. Hassler (Ed.). Coupling of Land and Water Systems . Ecol. Stud., University of Virginia, Charlottesville, 10, pp- 129- 136.
Phillips, E.A. 1959. Methods of vegetation survey. Holt, Rienhart & Winston Inc., New York.
Rao, A.N. & S. Deshmukh. 1994. In: Deshmukh, S.V. & V. Balaji (Eds.). Conservation of mangrove forest genetic resources: a training manual. ITTI-CRSARD project, M.S. Swaminathan Research Foundation, Chennai, India.
Ricklefs, R.E. & R.E. Latham. 1993. Global patterns of diversity in mangrove floras, in: R.E. Ricklefs & D. Schluter (Eds.). Species Diversity in Ecological Communities. University of Chicago Press, Chicago, pp- 215- 229.
Rojas-Beltran, R. 1986. Role de la mangrove comm. Nourricerie de crustaces et de poissons en Guyane. In: Sepanguy-Sep-Anrit (Ed.). Le Littoral Guyanais, fragilite de l'environment, ler Congres Sepanguy Nature Guyanaise, Cayenne, pp- 97- 110.
Saengar, P. 1996. Mangrove flora: Distribution of species and habitat descriptions. In: D.I. Walker, F.E. Wells, & J.R. Hawley (Eds.). Marine biological survey of the Eastern Kimberley, Western Australia. The University of W.A., W.A. Museum and the Museum of Art Gallery of the Northern Territory, Perth, pp- 39- 53.
Siddiqi, N.A. 1998. Enrichment planting in the mangrove of Sunderbans- Arcview, Bangladesh Journal of Forest Science 27: 103- 113.
Singh, D.K. & G.K. Panda. 1999. Bhitarkanika and its environs- a geographical appraisal, in: Bhitarkanika- the wonderland of Orissa. Nature and Wildlife conservation society of Orissa, Bhubaneswar, India, pp- 10- 18.
Snedaker, S.C. & J.G. Snedaker,.1984. The mangrove ecosystem: Research methods. UNESCO, Paris.
Spalding, M, F. Blasco & C.D. Field (Eds.). 1997. World Mangrove Atlas. Japan: The International Society for Mangrove Ecosystems. Cambridge Samara Pub. Co., Cambridge.
Tomlinson, P.B. 1986. The botany of mangroves. Cambridge Tropical Biology Series, Cambridge University Press, 413 pages.
Upadhyay, V.P., R. Ranjan & J.S. Singh. 2002. Human mangrove conflicts-the way out, Current Science 83: 1328- 1336.
Walsh, G.E. 1974. Mangroves: a review. In: R.J. Peinold & W.H. Queen (Eds.). Ecology of Halophytes. Academic Press, New York, USA, pp- 51- 174.
4