Introduction

What
is restoration when applied to dry-zone vegetation in Galapagos?
In this paper I will
argue that restoration should be seen as one element in a strategy
for conservation management, and use examples from Galapagos to
illustrate such a strategic approach to restoration of dry forest and
other semi-arid vegetation types in the islands. The main questions I
address are:
What can we learn
about vegetation restoration from Galapagos experiences?
What should we do to
ensure recovery from a situation of threat/degradation?
I argue that four
basic steps should be followed in a specific sequence, in order to
ensure restoration of damaged dry forest and other habitats. These
are:
1. Identify and
understand the threat and its effects: what has caused the habitat
change and how has it changed?
2. Remove or reduce
the threat.
3. Monitor the
effects of removing or reducing the threat.
4. Intervene further
only when necessary to ensure recovery.
It is the last of
these four steps which is often thought of as "restoration",
but I argue that the whole process of steps 1-4 should be seen
as restoration and that step 4 may frequently not be required.
Further, when it is required, Step 4 usually necessitates more
research (return to Step 1), to identify and understand additional
threat factors which need to be counteracted, and to determine life
cycle stages at which the additional interventions (Steps 2-3)
may need to be made. That is to say, Step 4 is simply a renewed cycle
of Steps 1-3.
DRY
ZONE VEGETATION IN GALAPAGOS
The vegetation of
Galapagos is determined largely by orogenic rainfall and is therefore
strongly zoned by altitude (Wiggins & Porter 1971). Although
other schemes have been used, I accept here five principal zones,
each of which can be divided into sub-zones based mainly on habitat
structure (e.g. forest, shrubland or herb-dominated communities). The
lowest of the five principal zones is the Littoral Zone, a narrow
fringe determined by proximity to the sea and the influence of salt
spray. Above this, the Dry Zone of scrub and dry woodland extends
uphill, where it blends into a Transition Zone of closed forest.
Above this is the Humid Zone, and finally on the highest islands,
above the main cloud layer, a second High-altitude Dry Zone.
The Galapagos
archipelago comprises 14 principal islands and more than 120 smaller
islets, where an island or islet may be defined as any landmass
permanently isolated (at all tide stages) by sea and capable of
supporting terrestrial vegetation (which excludes mangroves). All
Galapagos islands have a Littoral Zone, and some 50 islets are so
small and low that they are entirely Littoral Zone. All remaining
islands, i.e. about 80, have a Dry Zone, whereas only seven islands
are high enough to carry a Humid Zone and only two are high enough to
carry a High-altitude Dry Zone. The Dry Zone thus accounts for the
largest total land area of any of the main Galapagos vegetation zones
(Fig. 1).
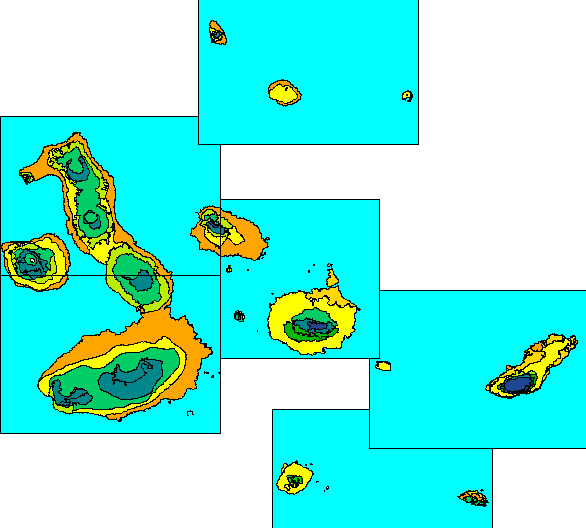
Figure 1. Dry Zone (yellows and oranges), Transition
Zone (greens) and Humid Zone (blues) of the central Galapagos
archipelago; High-altitude Dry Zone not distinguished on this map.
Ecological zone map of PRONAREG et al. (1987).
The vegetation
formations of the Dry Zone include various open woodland communities,
the most common being dominated by Bursera graveolens. Other
communities include more or less wooded shrubland dominated by
different species in different sites, including Cordia lutea,
Gossypium darwinii, Opuntia spp. or Croton scouleri,
among others. Other areas carry only sparse low shrubs and annual
herbs, while others are open lava with scattered annuals or cacti.
The Transition Zone is also semideciduous mixed-species Dry Forest
(Bosque Seco Premontano).
The Problem
Threats
to Galapagos vegetation
Galapagos vegetation
has suffered three main threats, direct habitat destruction by man,
direct exploitation of certain species, and introduced species.
Historically, direct
habitat destruction by man has been important, with the creation of
large agricultural areas in the highlands of four islands, and
settlements on the coasts of five. The lowland towns have damaged
only a tiny proportion of the widespread Dry Zone. In contrast, the
agricultural areas have destroyed large proportions of the Humid Zone
of Floreana, Santa Cruz and San Cristóbal, and of Sierra Negra
volcano on Isabela, and have also damaged substantial proportions of
the Transition Zone on these islands (e.g. Fig. 2; Snell et al.
2002/in press). There is
currently pressure and legal provision to take more land from the
Galapagos National Park for development in Galapagos. Depending where
this is done, it could have major or lesser effects on one or more
vegetation zones in the coming years.
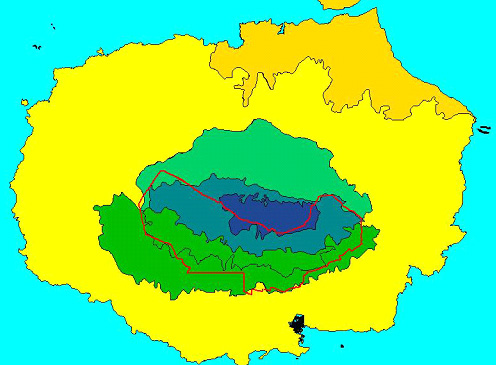
Figure 2. Impact of the conversion of natural habitats
to agricultural (red outline) and urban (town on south coast) use on
Santa Cruz Island. Habitat zones as in Fig. 1.
Direct exploitation,
although an important cause of declines and even extinctions of some
Galapagos animal species, such as giant tortoises and sea cucumbers,
has affected only a few plants. Some native and endemic trees have
been exploited for their timber, which may have caused population
structural changes, and in one case, the Floreana endemic tree Lippia
salicifolia, probably contributed to its current threatened
status (Mauchamp et al. 1998; Tye 2002). However, direct
exploitation has not contributed significantly to changes in the
structure or composition of the Dry or Transition Zones, except very
locally where mature Piscidia carthagenensis trees have been
over-exploited.
Introduced species
are currently regarded as the main threat to the biological diversity
of Galapagos (Bensted-Smith 2002/in press).
Invasive introduced species may be regarded as having two main
effects: competition with native species and predation on them. In
the case of effects on vegetation, predation is equated with
herbivory, while the main competitors for native plants
are invasive introduced plants (Fig.
3ab).

Figure 3a. Effects of competition (invasion of the tree Cinchona pubescens) in the naturally treeless highlands of Santa Cruz Island: photo H. Jäger).

Figure 3b. Effects of predation (= herbivory, destruction of dry forest on Alcedo Volcano by goats) by introduced species in Galapagos.
Restoration
required following different kinds of damage
From the above it
will be seen that each of the three main threats leads to a distinct
restoration requirement. Habitat destruction, conversion to
agricultural or urban use, may require considerable investment in
order to restore native vegetation, after any decision has been made
to remove the threat (i.e. to cease use and restore native habitat).
Most native plant species will have been eliminated from such areas,
so their return following cessation of agriculture, or dis-occupation
of the area, is highly unlikely, at least in a reasonable time frame.
Restoration following such use therefore implies intervention to
restore the entire plant community. Such restoration has not been
attempted on a large scale in Galapagos, although pilot projects have
been started in recent years in the agricultural zones, where some
land-owners have begun conservation-restoration projects in part
because of their potential value as an ecotourism attraction.
However, these projects all focus on humid-zone vegetation, although
some include upper Transition Zone areas, and none affects the Dry
Zone.
In contrast,
restoration following direct exploitation of individual species would
require intervention focused on those threatened species. So far,
there have been no attempts in Galapagos to restore the populations
of any timber tree whose populations have been affected by
over-exploitation. Management interventions have simply comprised
attempts to reduce use.
Restoration
following damage by introduced species is more complex, in that the
level of intervention required (community vs. individual species) is
intuitively less clear. However, restoration following damage by
introduced species is precisely the area where most research has been
done in Galapagos. Experiences and lessons learnt in Galapagos may
thus provide useful insights into the factors that need to be taken
into account when planning a restoration programme following this
kind of damage. This kind of restoration programme is the focus of
the rest of this paper.
Restoration steps following damage caused by competition from Invasive Plants
Step
1: Identify and understand the threat.
Although questions
such as "what damage are invasive plants causing?", are
also important in order to permit evaluation of the success of a
restoration programme (reversal of the changes), the principal
question to which an answer is required for planning restoration
following damage caused by invasive plants is "which species
are causing most damage?". From the point of view of
competition, invasive plant species may be classed as either
"integrators" or "transformers" (see
Richardson et al. 2000). Integrators, often but not always
small herbaceous species, may invade natural communities without
dominating them or displacing the majority of the native vegetation.
They are thus often difficult to control, but they also cause lesser
effects. Transformers in contrast are often large woody species, or
herbaceous species that can form dense stands, such as grasses. They
may thus displace the majority of the native vegetation and can
replace it with a totally alien community, in terms of both structure
(e.g replacing herbaceous communities with forest) and species
composition (displacing native species).
In the Humid Zone,
many invasive plant species have escaped from the agricultural areas
and are causing widespread, landscape-scale changes to the
vegetation. In the Dry Zone, invasive plants have not yet caused
serious damage, but they may do so in the future, as many potentially
invasive introduced species that are adapted to drier conditions have
been introduced as garden ornamentals and are grown in the lowland
towns. However, some invasive plants, such as Cedrela odorata,
Psidium guajava, Passiflora edulis and a variety of
grasses, are invading the Transition Zone on the inhabited islands,
spreading downslope from the agricultural areas and replacing native
dry forest. Monitoring projects and ecological studies help to
determine which invasive plants are transformers. For example in the
mixed forest of the Transition Zone of Santa Cruz island, a 30-year
monitoring programme of permanent plots has revealed the complete
replacement of the dominant tree Scalesia pedunculata by the
introduced Cedrela odorata and Psidium guajava within a
13-year period (1992-2004:
Fig. 4ab).

Figure 4a. Replacement of Transition Zone Scalesia
pedunculata forest by introduced Cedrela odorata and Psidium guajava. 1992. Photos: O. Hamann.

Figure 4b. Replacement of Transition Zone Scalesia
pedunculata forest by introduced Cedrela odorata and Psidium guajava. 2004. Photos: O. Hamann.
Step
2: Remove or reduce the threat.
The science of
invasive plant management is still relatively young, and there are
few examples of control of a plant invasion on a large scale in
Galapagos so far. In the case of the transformation of the Transition
Zone forests, a pilot project was undertaken to kill all Cedrela
trees over several hectares of heavily invaded forest. Two major
treatments were used, one where trees were felled and stumps killed
by painting herbicide on them, and the other where trees were killed
standing by injecting herbicide into machete cuts in the trunks. The
latter treatment avoided damaging the understorey caused by felling
trees, as the understorey included young plants of many native canopy
species. Both trials gave almost 100% control of Cedrela,
resulting in opening of the canopy, which had become dominated bythis
species. This was followed by strong regrowth of native vegetation,
but also by regeneration of Cedrela from seed (Fig.
5ab), indicating that follow-up treatment would be required to
ensure full restoration of native forest. However, the trials
indicated that it would be relatively easy to achieve restoration
over large areas, if sufficient funding could be found to employ a
small team to carry out initial control and follow-up maintenance.

Figure 5a. Former Transition Zone forest dominated by
the invasive Cedrela odorata (the tall trees).

Figure 5b. Former Transition Zone forest dominated by
the invasive Cedrela odorata, following Cedrela control. The red oval indicates Cedrela regrowth, among a general regeneration of native species.
Larger projects are
therefore needed in Galapagos to attack some of the worst invaders,
and the key to success of a large project is detailed planning and a
systematic approach. The best example of such an approach for
planning a large-scale control, or even total eradication, project in
Galapagos concerns Red Quinine, Cinchona pubescens, which has
invaded some 12,000 ha on Santa Cruz island, mostly in the humid
highlands but also extending to the Transition Zone.
If we are to begin
such a project with a high probability of succeeding, we need to
understand in great detail the biology of the target plant. This
project illustrates the deeper level of understanding required at
Step 1, once a target has been chosen. In the case of Cinchona,
studies have been carried out in Galapagos on its reproductive cycle,
seed dispersal and seedbank longevity (Rentería 2002) as well
as on control trials, the impacts of the invasion and the impacts of
control techniques (Jäger 1999). The biological studies are
necessary to enable a control project to interrupt the reproductive
cycle, prevent further seed production and permit steady reduction in
the population of juvenile plants (before they reach reproductive
age). Further studies have been carried out to estimate the cost of
control at different densities (Buddenhagen & Yánez
2005). Since the main determining factor for the success of such a
project is the availability of funds, it is important to be able to
predict the total cost of a project, especially if the goal is total
eradication from an island, in advance of beginning the attempt. All
of these studies contribute to a management plan for either total
eradication of the species from Santa Cruz or its control (reduction
to low density) over the whole of the invaded area.
Management options
for removing or reducing the threat caused by an invasive plant fall
into three main categories:
1. "Classical"
control, implying manual or chemical control where the aim is a
reduction in density of the target species over a defined area, in
practice usually specific sites considered to be of high biodiversity
value.
2. Eradication,
where the aim is complete removal of a species from a defined area,
and where the chance of re-invasion is small. In practice this
usually means eradication from an island.
3. Biological
control, where the aim is permanent reduction in density over the
entire invaded area.
Each of these
options carries advantages and disadvantages, and cost distributions
differ significantly between the three. In the case of classical
control, costs are primarily determined by the size of the selected
control area, which may in practice be determined by the size of the
annual budget assigned by the management authority. This results in
apparently lower costs, when compared with eradication or biological
control, but the disadvantage is that success is variable from year
to year (depending on variations in annual budget) and the investment
never ends, since the species requires continued control year after
year in order to maintain gains obtained by reducing the population
of the target species. In the case of Cinchona on Santa Cruz,
a suitable level of investment required to keep the most important
sites on the island relatively free of the invader would be between
US$100,000 and $300,000 per year (depending on the size of the area
selected for treatment), permanently.
Biological control
has a very different cost distribution, with initial investment high,
being mainly the costs of the research required to identify an
effective natural enemy, usually in the area of origin of the
invader, and further research needed to determine that the identified
agent will not have adverse effects on native plants or animals
(specificity testing). However, once implemented, if control is
successful, future costs reduce to near zero (low-frequency
monitoring to ensure that control remains effective). The
disadvantage associated with a new biocontrol project is that, until
the research has been done and the release of the agent has been
carried out, the ability to predict success is poor: an agent may or
may not be found, and the agent may or may not reduce the target
species' population to acceptable levels. In the case of
Cinchona, such a project would be much cheaper than most
biocontrol efforts, since it could be carried out entirely
within-country, as the natural range of the species includes mainland
Ecuador. The total cost would be c. $300,000.
Eradication, in
contrast, may appear extremely expensive, since initial costs, as in
biocontrol, are high, in this case not for the research, but for the
initial knock-down of the population to interrupt the breeding cycle.
However, as in the case of biocontrol, once the plant has been
eradicated, costs reduce to near-zero, and a further advantage of
eradication is that our ability to predict success is high, as long
as the preliminary studies have been thorough. Predicting success
largely comes down to predicting cost, the question then reducing to
ability to find and dedicate the funds required, and to guarantee
continuation of the project for the period required to achieve
eradication. In the case of Cinchona, the cost of attempting
an eradication from Santa Cruz with a high probability of success
would be c. $6 million (cf. Buddenhagen & Yánez
2005), spread over 10 years, with about half the total budget being
spent in the first three years (initial removal of all seed-producing
trees over the entire invaded area).
Although eradication
may thus appear prohibitively expensive, it may be the preferred
option, given that future costs are effectively zero. It is far
easier to guarantee a one-off investment of $6 million than to
guarantee a defined level of funding for classical control, every
year, for ever. Similarly, on economic grounds, biological control is
always a cost-effective option for dealing with a serious, widespread
invasion.
Restoration steps following damage caused by Herbivory
Step
1: Identify and understand the threat.
It is sometimes very
easy to detect the effects of introduced herbivores on native plant
communities and individual species. Introduced mammalian herbivores,
especially feral goats Capra hircus, are notorious for their
dramatic effects on oceanic islands (Baker & Reeser 1972; Breckon
2000; Coblentz 1978; Courchamp et al. 2003; Gould &
Swingland 1980; Lever 1994), and are often blamed for vegetation
change based on anecdotal (but obvious) evidence. Effects such as
extinctions, rarity and changed species composition have been
inferred based on qualitative comparisons with old accounts or
photographs, while monitoring studies have examined regeneration
following goat exclusion or eradication (e.g. Baker & Reeser
1972; Loope & Scowcroft 1985; North & Bullock 1986; all
studies cited by Coblentz 1978). In Galapagos, Hamann (1975, 1979,
1993, 2001) documented goat damage on Santa Fe and Pinta islands, and
De Vries & Calvopiña (1977) on Santiago. All these studies
demonstrate dramatic changes in communities, and declines in
individual species caused by goats.
Step
2: Remove or reduce the threat.
Solutions to such
problems include protection, such as constructing exclosures around
threatened communities or remnant populations of individual species.
Both community- and species-focused fencing has been used in
Galapagos. Fences were built around remnant vegetation communities on
Santiago Island during the period 1973-1998, when goats
devastated the vegetation throughout the island. Fences have also
been constructed to protect populations of threatened plant species,
such as Linum cratericola on Floreana Island, Cyathea
weatherbyana on Alcedo Volcano of Isablea Island, and Scalesia
retroflexa on Santa Cruz.
The advantage of
fencing is that it is relatively easy, quick and cheap, but can only
be used (at least while remaining cheap) to protect relatively small
areas. Over larger areas other alternatives become more
cost-effective, including control or eradication of the herbivore. As
in the case of invasive plants, there are three management options
for introduced herbivores: "classical" control (e.g.
hunting), biological control and eradication.
As with plants,
classical control can have lower annual costs, but is for ever, and
success is variable, depending on variations in annual budget and
priorities. Biological control has a high initial cost but later
falling to near-zero, success prediction is uncertain, and biocontrol
is in practice useful for only invertebrate animals. Eradication
costs are initially high, but then reduce to near-zero (monitoring
for reintroduction), and the prediction of success is high, at least
for mammalian herbivores.
As an example, goats
have been introduced to 12 Galapagos islands, and have so far been
eradicated from seven of these (Fig.
6). Eradication projects are almost complete for one more
(Santiago) and for the northern part of Isabela. Our successes are
improving, with ever larger islands being freed from goats.
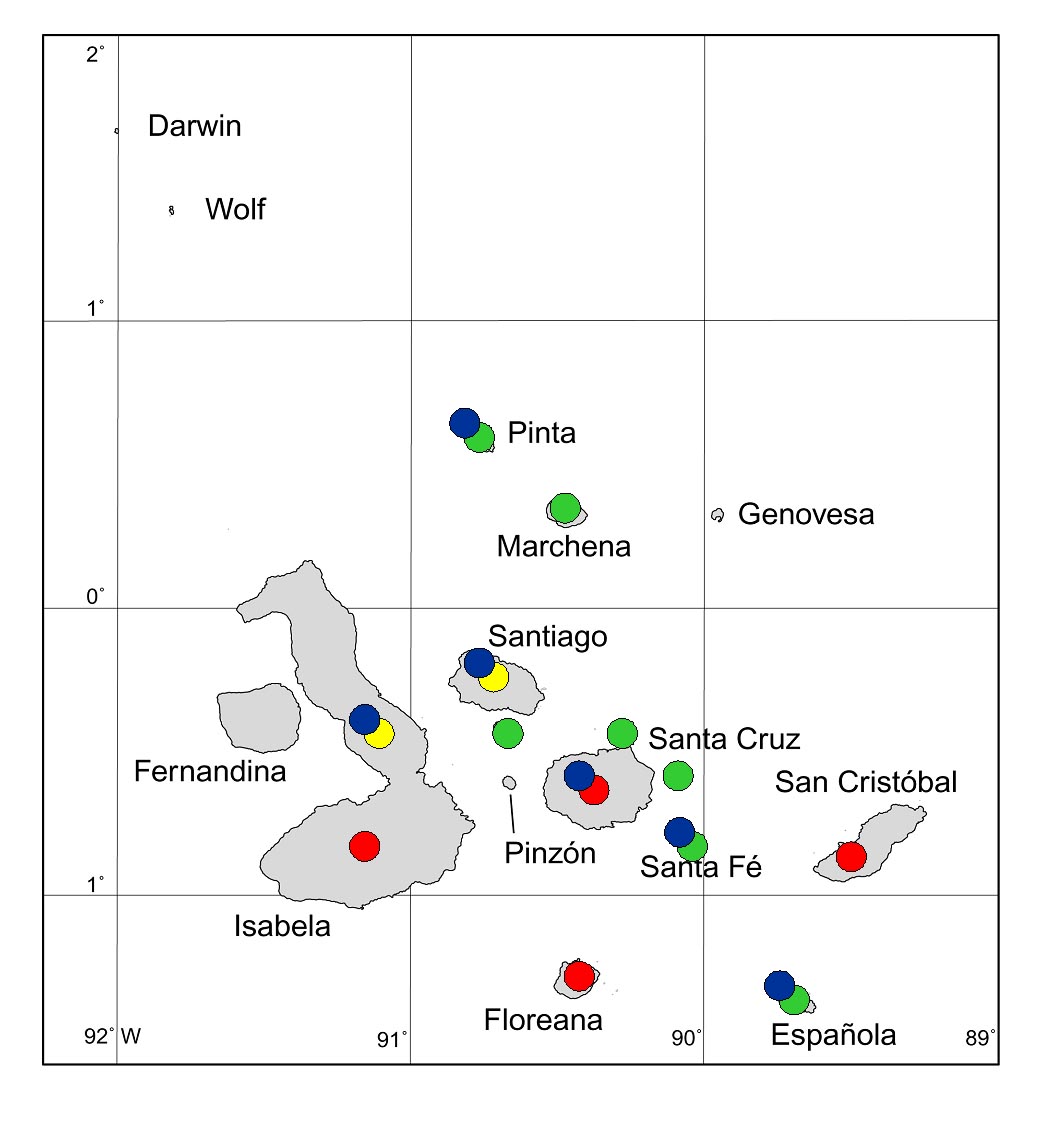
Figure 6. Galapagos islands to which goats have been
introduced and gone wild. Red circles = not yet eradicated; green = already eradicated; yellow = eradication in progress (as at end of 2005); blue = long-term vegetation monitoring projects in place.
Step
3: Monitor the effects of removing or reducing the threat
The importance of
monitoring both success of and impacts of control cannot be
over-emphasized. This is particularly true in the case of
eradication, where we need to be sure that all individuals of the
target invasive plant (including viable seeds) or animal have really
been removed. The importance of monitoring the impact of control lies
in determining whether the effect on native species and communities
is the desired one (e.g are native species regenerating in
appropriate proportions or are other invasive introduced species
replacing them?), and thereby detecting whether any further
intervention (Step 4: "restoration") is required: it may
not be. Once again, the example of goat eradications on Galapagos
illustrates this.
On six of the 12
islands where goats had been introduced, long-term vegetation
monitoring projects based on permanent plots and transects have been
established, which have so far gathered data for up to almost 40
years (Fig. 6). Goats were
eradicated from Santa Fe and Española in the 1970s, and these
have since been monitored by Hamann (1979, 2001) and H. Adsersen
(unpublished). Hamann and Adsersen have also monitored plots on Pinta
(Hamann 1975, 1979, 1993), from which goats were almost eradicated in
the 1970s, and then finally eradicated in 2003. Hamann (2001) has
also been monitoring plots on Santa Cruz, where goats have been
subject to varying levels of control but not eradicated; these plots
also demonstrated the changes brought about in Transition Zone forest
by Cedrela odorata and Psidium guajava. Many of these
island studies were initially established by De Vries (e.g. 1977, De
Vries & Calvopiña 1977), who also began
monitoring on Santiago, from which goats were not eradicated until
2005. Since the studies of De Vries, successive CDRS botanists have
continued monitoring on Santiago, and have established plots in 1995
on Alcedo Volcano (Isabela), from which goats may be eradicated in
2006.
These projects have
demonstrated that goat removal is usually followed by rapid
regeneration of native vegetation, attaining a structure and species
composition closely similar to the pre-goat state within 20 years.
The Galapagos flora, as is typical of oceanic islands, consists
largely of species with pioneer characteristics and has proved
remarkably resilient following removal of introduced species threats.
In other words, little further intervention (Step 4 restoration) is
usually required, beyond threat removal. We have several lines of
evidence that this is so.
For example on
Española, photographs taken in 1905-6 by the California
Academy of Sciences in various parts of the island demonstrate the
state of the vegetation shortly after the introduction of goats,
while transect comparisons between Española and its offshore
islet Gardner (which never harboured goats) show that the state
attained on the main island 20 years after goat eradication is
comparable with that of a nearby area unaffected by goats. On
Santa Fe, the species-focused studies of Hamann showed that Scalesia
helleri (Fig. 7) and
Opuntia echios, which had been badly affected by the goats,
regenerated more or less rapidly following eradication of the
animals.

Figure 7. Scalesia helleri regenerated rapidly
following the eradication of goats from Santa Fe island.
Exclosures
also show similar effects on a smaller scale. The only known
population of Linum cratericola increased from a low of 13
known plants to more than 400, when most of the population was
protected by fences, and goats and feral donkeys were controlled in
the surrounding area (Simbaña 2002 and unpublished).
The
general conclusion is that most Galapagos vegetation recovers fast
once a threat is removed, but does it all? The importance of careful
monitoring is demonstrated by cases where individual species have not
recovered following goat eradication or exclusion. Three examples
include Cyathea weatherbyana on Alcedo, whose last two remnant
populations were protected by fences in 1997, and Opuntia
megasperma and Lecocarpus lecocarpoides on Española,
where goats were eradicated in 1978. Such cases
lead us to Step 4 of the restoration process.
Step
4: Further intervention, when necessary to ensure recovery.
In
all these cases, the problem is with individual species, rather than
failure of a whole community to return to something close to its
original state. We therefore require autecological studies to
determine what further action needs to be taken to ensure their
recovery. Such studies must focus on identifying the additional
threat(s) which may be preventing recovery, and identifying the life
history stage(s) at which action must be taken to reverse the effect
of each threat. Cyathea weatherbyana on Alcedo, and Opuntia
megasperma and Lecocarpus lecocarpoides on Española
illustrate different stages in this process.
In
the case of the Cyathea, it was thought in 1996 that its
decline was associated with a process of dramatic vegetation change
on the volcano caused by a goat population explosion there in the
early 1990s (Cayot & Snell 1996). The planned solution to these
changes was to be an attempt to eradicate goats from northern Isabela
but, pending a search for funds for such an ambitious project, a
temporary measure intended to save the last tree ferns was the
fencing of the two remnant groups of plants, which was accomplished
in 1997. The two patches were included in the monitoring programme
that was begun in 1995. This has shown a continued decline in the
species, despite the fence. It has proved difficult to ascertain the
reasons for this continued decline. Contributory factors could
include occasional breaches of the fence by small groups of goats,
which have remained inside the exclosure for periods up to 2-3
months, but these were not closely correlated with population decline
of the tree fern. It has not been possible to initiate a detailed
study of the remaining plants (or of healthier populations on the
other islands), but periodic observations suggest that the plants may
be suffering water stress, as they appear to be almost permanently in
a semi-wilted state (Fig. 8).
This leads to the suspicion that micro-climatic changes as a result
of general loss of forest cover in the surrounding areas may be at
least partly responsible. Goat eradication finally began in 2003 and
at the end of 2005 was virtually complete. Results of monitoring on
Santiago Island show that highland vegetation recovery has been
astonishingly fast there following goat eradication, with a moderate
shrub-tree canopy expected to be reestablished over large areas
within 3-4 years. The strategy for Alcedo for the time being is
therefore to await the results of the goat eradication there, which
is about one year behind that on Santiago, to see if general
vegetation recovery promotes regeneration of the tree fern.

Figure 8. Remnant adult Cyathea weatherbyana tree
ferns inside a goat exclosure on Alcedo Volcano, Isabela Island.
Lecocarpus
lecocarpoides is still a common species on the four islets in
Gardner Bay, Española. On the main island it is known only
from a tiny population on the north coast (Fig.
9), which fluctuates between zero and 60 plants, behaving as
an annual. Since goat eradication in 1978, this population has not
increased and it is not known what restrictions might be preventing
an increase. In this case we have no evidence that the species was
more common on the main island before the introduction of goats, but
suitable habitat seems to be present in several parts of the island.
This species has not been the subject of any detailed study so far,
nor of any management intervention to try to increase its population.
However, a study is planned to examine seed germination and habitat
requirements, as a preliminary to an attempt to establish a
population at a second site on the main island.

Figure 9. A member of the tiny Lecocarpus lecocarpoides population on Española Island.
We
have more information about Opuntia megasperma, the other
Española species which has not increased since goat
eradication. This species may be used to illustrate the kind of
research that needs to be done to address such a problem.
First,
we have better evidence of the former status of Opuntia on
Española. The CAS photographs of 1905-6 show that large
adults were present in many parts of the island where no Opuntia
can be found today, including the eastern extremity, Punta
Cevallos. The nearest remaining Opuntia to Punta Cevallos are
now some 4 km away, making its return to such sites extremely
unlikely in the medium term. Second, we know that, even in areas
where adult Opuntia remain, regeneration has been poor or
absent (Grant & Grant 1989, Coronel 2002). We have therefore
carried out a series of studies to try to understand why.
Our
first hypothesis was that seed production or viability might have
been reduced, perhaps as a result of a genetic bottleneck, or that
seed survival might have been adversely affected by an imbalance
between the Opuntia population and those of its seed predators
(such as Darwin's finches Geospizinae), resulting in excessive
seed predation. Preliminary observation confirmed, however, that
adult plants were fruiting normally and that the fruits contained
normal numbers of seeds. Neither did seed survival appear to be the
problem, since abundant seed could be found on the ground in areas
where adult plants still occurred. Further, seed collected from the
ground and from fruits was viable. Laboratory experiments gave
germination rates of c. 40% with no pre-treatment, while seed
collected from the faeces of giant tortoises, after the fruit were
fed to them, gave c. 80% germination. Tortoises are natural
dispersers of Opuntia seed in Galapagos.
It is still not
known whether failure of germination in the field may be a factor
contributing to the lack of recovery. We know that germination was
high on Española in moderately wet years in the 1970s,
although perhaps poorer in exceptionally wet years in the early 1980s
(Grant & Grant 1989). It may be that germination requires wetter
than average conditions, and Galapagos has received below average
rainfall during the years 2002-5. However, no significant
regeneration has occurred since the 1970s, during which period a
broad range of Galapagos climatic conditions has been represented,
from very dry to very wet years. Some factor may therefore have got
worse over the intervening period. The presence of some seedlings in
limited areas shows that at least some natural germination has
occurred in recent years, and further monitoring under different
rainfall conditions may help to clarify the role of germination
problems.
However,
knowing that viable seed production is not the key factor, the
question passes to the next life stage: the seedling. The hypothesis
here was that seedling survival may have been compromised by lack of
water, predation, or other factors. We therefore cultivated seedlings
in the laboratory, acclimatized them to field conditions, then
planted them out in the field and gave them a variety of
post-planting treatments, comprising: given water and protected by
small cages; not given water but protected; protected but not given
water; and unprotected unwatered (Fig.
10). These experiments showed that water was not a critical
factor, but that protection by small cages dramatically improved
seedling survival (Coronel 2002). Observations indicate that native
animals on Española damage Opuntia seedlings in various
ways, either eating them or physically damaging them. Further
experiments are being carried out to determine which animals cause
important damage, and from which the seedlings require protection.
The problem may therefore be, at least in part, an imbalance between
the Opuntia (seedlings) population and the populations of its
seedling predators. A dramatic increase in the population of one of
the animals known to damage young Opuntia, the giant tortoise
on Española, is a result of another, highly successful,
Galapagos restoration programme. A
possible lesson for restoration planners is that
this may have contributed to the hypothesized worsening conditions
for the seedlings since the 1970s.

Figure 10. Research student Vanessa Coronel with Opuntia megasperma seedlings planted in the field on Española Island.
A
final Galapagos example serves to illustrate a case where Step 4 is
inevitable: where removal of a threat cannot result in recuperation
of a population without further intervention. This is the case where
the population is extinct, either locally or completely in the wild.
Scalesia atractyloides, endemic to Santiago Island, exists in
a number of distinct populations some of which are isolated by
unsuitable intervening habitat. Some of the populations show
distinctive morphological characteristics, so each is probably
genetically valuable. The species was greatly reduced by goats, but
most of its populations are expected to recover now that the goats
have been almost eliminated from the island. However, at least one
population, at Ladilla Bay (Fig.
11ab), became extinct in the 1980s or 1990s, but not before
seed from this population had been taken into cultivation in
Copenhagen Botanic Garden. After the goats have been confirmed
eradicated from Santiago, we intend to use seed from Copenhagen in an
attempt to re-establish this population.

Figure 11A. Ladilla Bay, Santiago Island, former site
of Scalesia atractyloides.

Figure 11B. Scalesia atractyloides.
Conclusions

Restoration Messages from the Islands
"Restoration"
may be seen in its more restricted sense as a part of a conservation
strategy, or the term may be used to define that strategy. In this
broader sense, restoration includes three main steps, understanding
the threat, removing or reducing the threat, and observing the
result. If the required result is not obtained, then further
intervention (restoration in its more restricted sense) may be
required.
However, examples
from Galapagos show that in the case of an oceanic archipelago, with
a flora with pioneer characteristics, the vegetation is often
sufficiently resilient that this final or additional step is not
required. Where the threat factor is an introduced species, simply
removing that species may be enough to ensure full recovery of the
native vegetation, especially on islands where other introduced
species are not available to replace the first.
On the other hand,
when further intervention is required, this comprises in effect a
return to the original three steps: identifying and understanding
additional threats, removing or reducing those, and watching what
happens. Further, in contrast to the first round of the three initial
steps, where this final step (or new cycle of steps 1-3) is
required, it usually requires action directed at an individual
threatened species, whereas the first round usually deals with a
threat that affects an entire vegetation community. The kinds of
ecological studies and monitoring required thus differ completely in
a second round of "restoration", where a species focus is
appropriate, from those required in the first round, where the focus
is on vegetation communities.
Acknowledgements

I
would like to express my profound gratitude to my colleagues and
predecessors in the CDRS Botany Department for their hard work over
the years on the projects mentioned in this article, and for the use
of their photographs. Unacknowledged photos are my own. This is
Contribution 1030 of the Charles Darwin Research Station.
References

Baker J.K. &
D.W. Reeser. 1972. Goat management problems, Hawaii Volcanoes
National Park. Natural Resources Reports 2, US Dept of the
Interior National Park Service.
Bensted-Smith, R.
(ed.) 2002 (online); in press (2006) as
book. A Biodiversity Vision for the Galapagos Islands.
Charles Darwin Foundation and WWF, Puerto Ayora. Online at:
http://www.darwinfoundation.org/downloads/bio_vision_galapagos_eng.pdf
Breckon G.J. 2000.
Revision of the flora of Desecheo Island; Puerto Rico. Caribbean
Journal of Science 36: 177-209.
Buddenhagen, C. &
P. Yánez. 2005. The cost of Quinine
Cinchona pubescens control on Santa Cruz Island, Galapagos.
Galapagos Research 63: 32-36.
Cayot, L. & H.M.
Snell. 1996. Goats damage Volcán Alcedo,
Isabela. Noticias de Galápagos 56: 3.
Coblentz, B.E. 1978.
The effects of feral goats (Capra hircus) on island
ecosystems. Biological Conservation 13: 279-286.
Coronel,
V. 2002. Distribución y re-establecimiento de Opuntia
megasperma var. orientalis Howell. (Cactaceae) en Punta
Cevallos, Isla Española - Galápagos. Tesis de
grado, Universidad del Azuay, Cuenca, Ecuador.
Courchamp
F., J.-L. Chapuis & M. Pascal. 2003. Mammal invaders on
islands: impact, control and control impact. Biological Reviews
78: 347-383.
De
Vries, T. 1977 Como la caza de chivos afecta la vegetación en
las islas Santa Fé y Pinta, Galápagos. Revista de la
Universidad Católica 16: 171-181.
De
Vries, T. & Calvopiña, L.H. 1977. Papel de los chivos en
los cambios de la vegetación de la isla San Salvador,
Galápagos. Revista de la Universidad Católica
16: 145-169.
Gould, M.S. &
I.R. Swingland. 1980. The tortoise and the goat: interactions on
Aldabra island. Biological Conservation 17: 267-279.
Grant,
B.R. & P.R. Grant 1989. The slow
recovery of Opuntia megasperma on Española. Noticias
de Galápagos 48: 13-15.
Hamann, O. 1975.
Vegetational changes in the Galapagos Islands during the period
1966-73. Biological Conservation 7: 37-59.
Hamann, O. 1979.
Regeneration of vegetation on Santa Fé and Pinta Islands,
Galápagos, after the eradication of goats. Biological
Conservation 15: 215 - 236.
Hamann, O. 1993. On
vegetation recovery, goats and giant tortoises on Pinta Island,
Galapagos, Ecuador. Biodiversity and Conservation 2: 138-151.
Hamann, O. 2001.
Demographic studies of three indigenous stand-forming plant taxa
(Scalesia, Opuntia, and Bursera) in the
Galapagos Islands, Ecuador. Biodiversity and Conservation 10:
223-250.
Jäger,
H. 1999. Impact of the introduced tree Cinchona pubescens Vahl
on the native flora of the highlands of Santa Cruz Island (Galapagos
Islands). Diplomarbeit, University of Oldenburg, Germany.
Lever,
C. 1994. Naturalized Animals. Poyser, London.
Loope,
L.L. & P.G. Scowcroft. 1985. Vegetation response within
exclosures in Hawai'i: a review. Pp. 377-431 in: C.P.
Stone & J.M. Scott (eds) Hawai'i's Terrestrial
Ecosystems: Preservation and Management. University of Hawaii,
Manoa.
Mauchamp,
A., I. Aldaz, E. Ortiz & H. Valdebenito. 1998. Threatened
species, a reevaluation of the status of eight endemic plants of the
Galápagos. Biodiversity and Conservation 7: 97-107.
North,
S.G. & D.J. Bullock. 1986. Changes in the vegetation and
populations of introduced mammals of Round Island and Gunner's
Quoin, Mauritius. Biological Conservation 37: 99-117.
PRONAREG, ORSTOM &
INGALA 1987. Islas Galápagos. Mapa de
formaciones vegetales. PRONAREG, ORSTOM, INGALA, Quito.
Rentería,
J.L. 2002. Ecología y manejo de la Cascarilla (Cinchona
pubescens Vahl), en Santa Cruz, Galápagos. Tesis de Grado,
Universidad Nacional de Loja, Ecuador.
Richardson,
D.M., P. Pyek, M. Rejmánek, M.G. Barbour, F.D. Panetta &
C.J. West. 2000. Naturalization and invasion of alien plants:
concepts and definitions. Diversity and Distributions 6:
93-107.
Simbaña,
W.A. 2002. Ecología y biología reproductiva de Scalesia
atractyloides Arnott, Scalesia stewartii Riley
(Asteraceae) y Linum cratericola Eliasson (Linaceae), especies
endémicas amenazadas en Galápagos. Tesis de grado,
Universidad Central del Ecuador, Quito.
Snell, H.L., A. Tye,
C.E. Causton & R. Bensted-Smith. 2002
(online); in press (2006) as book. The status of and
threats to terrestrial biodiversity. Chapter 5 in A Biodiversity
Vision for the Galapagos Islands. Charles Darwin Foundation and
WWF, Puerto Ayora. Online at:
http://www.darwinfoundation.org/downloads/bio_vision_galapagos_eng.pdf
Tye A. 2002.
Revision of the threat status of the endemic flora of Galapagos.
Galapagos Report 2001-2002. pp
116-122. WWF - Fundación Natura, Quito.
Wiggins, I.L. &
D.M. Porter. 1971. Flora of the Galápagos Islands.
Stanford University Press. Stanford, USA. Fig 5 both photos M.
Gardener, Fig 11 right photo P. Jaramillo











